HEISENBERG UNCERTAINITY PRINCIPLE
Heisenberg uncertainity principle state that, “It is impossible to determine position and momentum of electrons simultaneously with greater accuracy.” It is impossible to determined position and momentum of electrons because;-
(i)The size of electron is very small and as such radiations of high energy extremely small wavelength are required to detect it.
(ii) Impact of these high energy photons changes both the direction and speed of the electron.
Thus; the very act of measurement disturbs the position of electron. The uncertainties in the determination of these two quantities vary inversely. If one is determined fairly
accurately, the other must be corresponding less accurate. The distance of electron: Is a position of electron from the nucleus and momentum of electrons is product of mass of
electrons and velocity of the electron, Heisenberg put forward an expression which is used to determine uncertainity position and momentum of electrons.
Where: P – uncertainity momentum
X – Uncertainity position
P 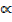
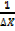
P = x 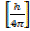
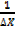
P = 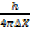 …………………. (1)
…………………. (1)
Since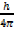 = proportionality constant
= proportionality constant
Also
mc =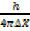 …………………. (2)
…………………. (2)
Example
The uncertainity in the momentum of particles is 3.3 x 10-16gms-1. Find accuracy with which its position can be determined
X = 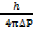
X = 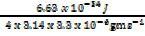
= 1.59 x 10-29m
WAVE MECHANICS
Wave mechanics deal with electron occupying space. The electrons revolve the nucleus through orbit and occur in the orbital.
Orbital:Is a region within an atomic sublevel that can be occupied by maximum two electrons that have opposite spin. Any orbital can accommodate a maximum of two electrons.
The energy level of atoms is a specific region around the nucleus electrons can occupy in atoms. A shell is defined as a complete group of orbital possessing the quantum number. The
electrons arranged in the atom starting from orbit from nucleus toward the orbit of highest energy level. The following include position of locating electrons in the atom.
i. Principal quantum number (n)
The atom shells called principal quantum number (main energy level). The electrons filled in atom in order of increasing the energy from the nucleus. Each shell have specific constant number or electrons obtained taking
e = 2n2
(ii)Subsidiary quantum number (L)
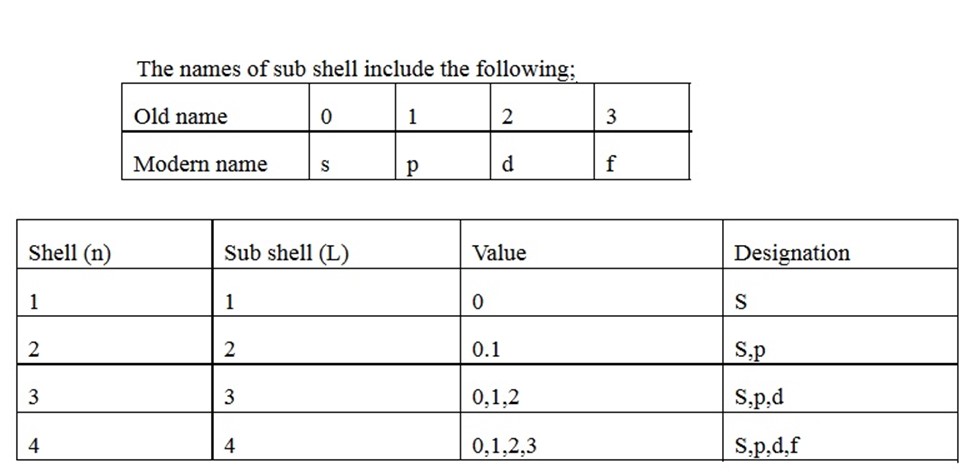
Subsidiary quantum number is a sub energy level. The sub energy level known as azimuthal quantum number, sub shell or sub energy level. The shell divides to form sub shell. The total number of sub shells in each shell is equal to the number of shell from the nucleus. This describes shapes of orbitals (sub-energy level).Values of subsidiary quantum number start from 0——-n-1
The names of sub shell include the following;
iii. Magnetic quantum number (m)
This is a quantum number which describes the orbital of each sub shell. This sub shell divided and form orbital. Each sub shells have specific number of orbital.
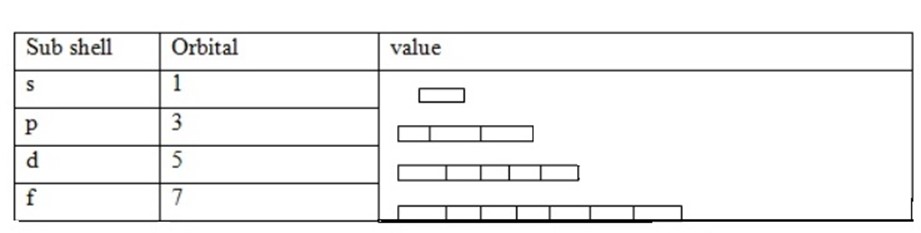
(iv) Spin Quantum number (s)
These quantum numbers describe the spinning of electrons in the orbital. Maximum number of electrons in the orbital in only two which spines in opposite direction. Since each orbital has two maximum electrons result the sub shell to have constant total number of electrons which accommodated in all shells S2, p6, d10, f14, g18, h22 and i26. In order to write electrons of element consider the knowledge of all four quantum numbers.
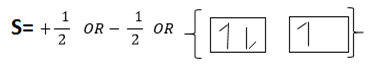
The following series of sub shell used to write the electrons structure.
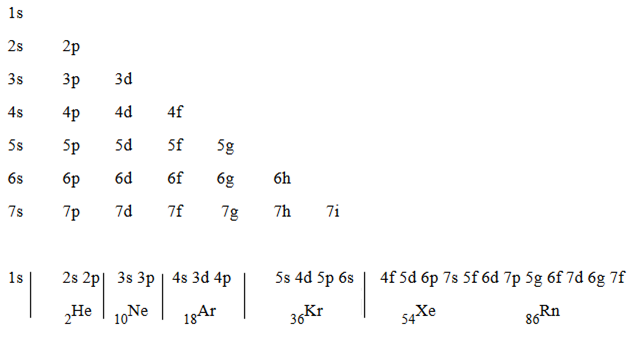
This arrangement used to write the electronic configuration.
Arrangement used to write the electronic configuration has some irregularities or abnormality. This irregularity occurs because there is overlapping of orbital. The orbital of low energy jump toward highest energy level and orbital of high energy drop down towards lowest energy level. Example 4s arranged first before 3d orbital.
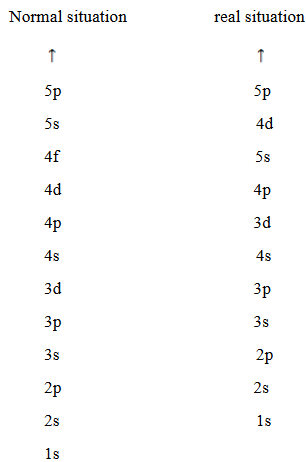
RULES WHICH GOVERN ARRANGEMENT OF ELECTRONS IN ORBITALS
1. Aufbau’s principle – State that “in the ground state of orbital the lowest energy is filled by electrons before filling orbital of the highest energy“.
This means that electron filled in order of increasing energy level. The orbital of lowest energy when is full of electrons then the electron filled on the next orbital of highest energy level. Consider the following arrangement
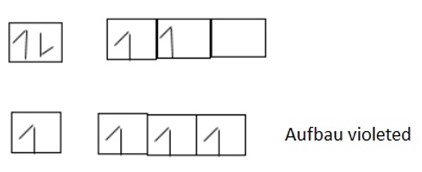
2. Hund’s rule of maximum multiplicity
State that, “The electrons pair in the orbital of the same sub shell is allowed if all orbital of the same energy are occupied by electrons of maximum multiplicity. This means that the degenerate orbital are not allowed to pair up unless each orbital is singly occupied. The incoming pairing is done in an opposite spin. Consider a case with 5 electrons in valency shell.
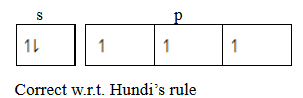
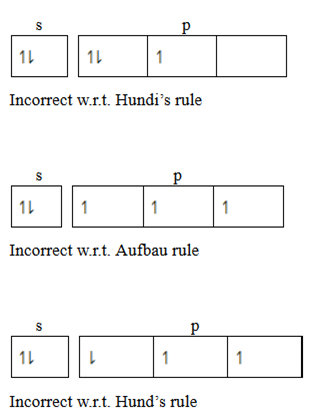
3. Paul’s exclusive principle
States that, “Two electrons in an atom can never have exactly similar set of all quantum numbers.” This means that the maximum number of electrons which is occupied in the orbital is only two. Two electrons may occupy the same orbital only on condition that they have their spin in the opposite direction. Two electrons may have three quantum numbers the same; n, l and ml but the fourth, ms must be different.

Sometimes the electrons structure or electronic diagram of ions is written according to what kind of ions given.
There are two kinds of ions which include the following;-
Cation or metallic ion is formed through losing of electrons. The electrons which are lost occur in the orbital out the of the noble gas structure. The electron removed starting from orbital of low energy toward the orbital of highest energy.
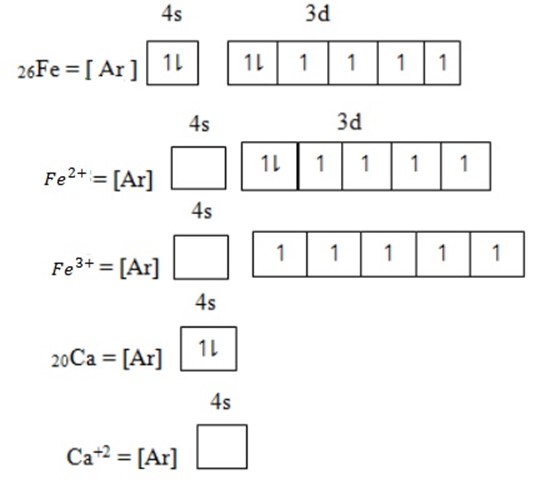
Anion or non-metallic ion – is formed by gaining electrons. Electrons which gain is added in the orbital having unpaired electrons starting from orbital of lowest energy to the orbital of highest energy level
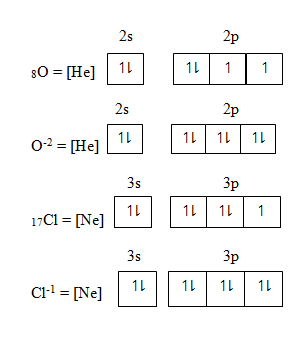
QUANTUM NUMBER
Is a number which describe the characteristics of electrons or is a number which is used to characterize electrons as they occupy orbitals in different energy levels. The quantum number is a number which describe main energy level, orientation of orbitals and spinning of electrons.
There are four type of quantum number which includes the following:-
1.Principle quantum number (n): Is a number which specifies the location and the energy of electron. This is a number which describe the main energy level of electrons. The principle quantum number called shell orbit, main energy level and stationary state. The principle quantum number have specific name from the nucleus. K, L, M, N etc.
2. Subsidiary principle quantum number (L): Is a number which specifies the shape of orbitals. Subsidiary principle quantum number is a sub energy level. The sub energy level is known as azimuthal number sub shell or sub energy level. The shell is divided to form sub shell. The total number of sub shells in each shell is equal to the number of shells from the nucleus. The names of sub shells include the following; s, p, d, f, g, h, i. The value of sub energy level i.e. 0, 1, 2, …L = n – 1.The value of L must be smaller that n because L = n – 1
|
n |
Value of L |
Sub shell |
|
1 |
0 |
1s |
|
2 |
0, 1 |
2s2p |
|
3 |
0, 1, 2 |
3sp3d |
|
4 |
0, 1, 2, 3 |
4s4p4d4f |
|
5 |
0, 1, 2, 3, 4 |
5s5p5d5f5f |
edu.uptymez.com
3. Magnetic quantum number: Is a number which specifies the number of orbital present in a given value of subsidiary quantum number. This is a quantum number which describes the orientation of orbital. The magnetic quantum number is a number of orbital. When the sub shell divides form orbital, these orbitals are called Magnetic quantum number.
1s – Orbital S = X
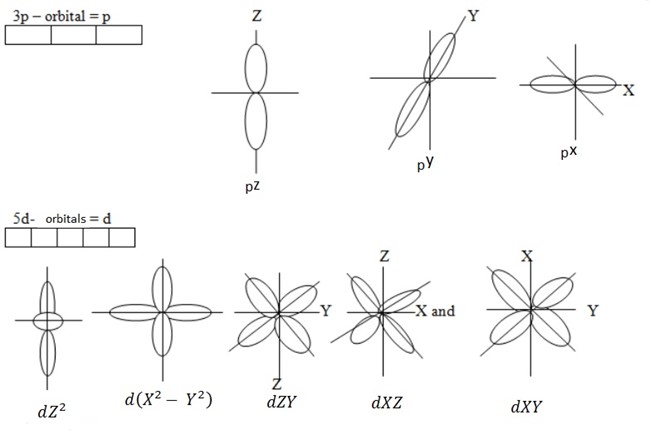
4. Spin quantum number (s) – This is a quantum number which describes the direction in which the electrons spin. There are two maximum electrons, these electrons are moving in opposite direction. They revolve clockwise + ½ and another revolve anti – clock wise – ½
APPLICATION OF QUANTUM NUMBER
Quantum number is applied to find the number of sub shell (L), number of orbital (m) and total number of electrons (s) of the given quantum number (n). There are two methods which are applied to find total sub shells, orbital and electrons.
Find total number of electrons in the principle quantum number 2.
(By using tree diagram)
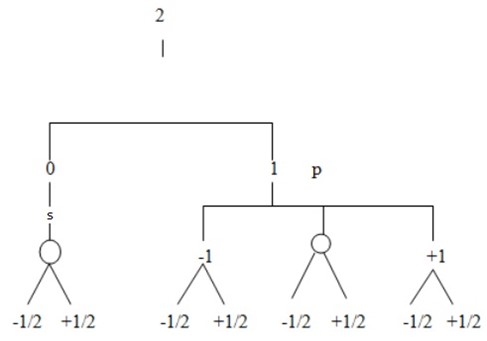
Total shells = 2 (s, p)
Orbitals = 4 (0, 0, +1, -1)
Electrons = 8
Also: by using tabular form
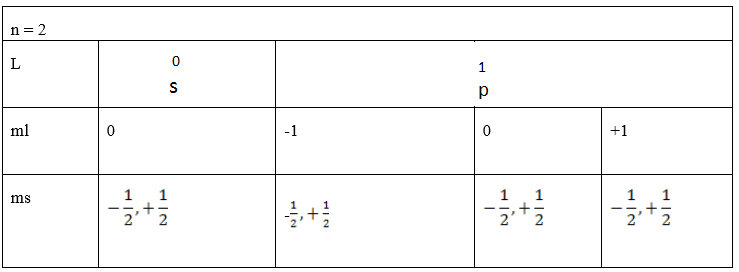
(b) Find the total number of electrons in the principle quantum number 3
Soln: (by using tree diagram)
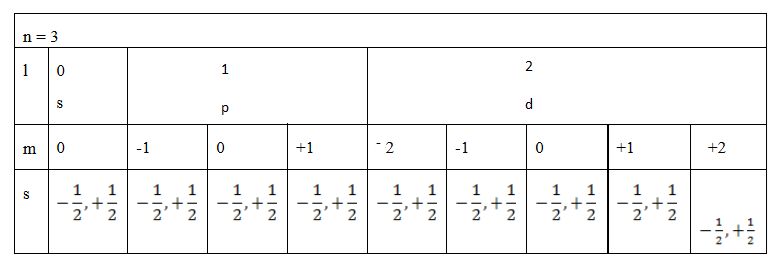
Example
(a) The modern theory of electron behavior is based on two assumptions. State them
(b) Define the following terms
i. Orbital.
ii. Energy levels of atoms.
iii A shell.
iv. Quantum.
Solution:
(a) The modern theory of electron behavior is based on two assumptions.
(i) An electron has a dual nature i.e. it behaves both as a particle and wave.
(ii) It is practically impossible to determine simultaneously both the position and momentum of an electron with any degree of precision.
(b) (i) An Orbital is a region within an atomic sublevel that can be occupied by a maximum of two electrons that have opposite spin. There are s,p,d, and f orbitals is a volume of space surrounding the nucleus within which there is a more than 95% chances of finding electrons. Any orbital can accommodate a maximum of two electrons.
(ii) Energy level of atoms in specific region around the nucleus that electrons can occupy in atoms.
(iii) A shell is defined as a complete group of orbital possessing the same quantum number.
(iv) Quantum is the smallest countable, discrete packet or increment of radiant energy that can be absorbed or emitted.
Example
Explain the meaning of the following terms:
(a) Quantization of energy and radiation.
(b) Wave particles duality of matter.
(c) Quantum number.
Solution
(a)Light and other forms of electromagnetic radiations are not emitted continuously but in discrete “packets” called photons. Energy of an electromagnetic radiation is also not emitted continuously but in “packets” called quanta. A photon of radiation carries a quantum of energy. Energy and radiation are quantized in that electron shall fall from one energy level to another and not anywhere else in between.
(b) According to de Broglie, every sub – atomic particles has both wave and particle properties. Photons of light and other electromagnetic radiations, are regarded and wavelengths. They are regarded as waves because they have specific frequencies. Although electrons have extremely small mass, they have both velocity and momentum as particles.
(c) Quantum numbers are used to characterize electrons as they occupy orbitals in different energy levels. Each electron is characterized by four quantum numbers:-
The principal quantum number, n, which specifies the location and energy of the electron.
The azimuthal, subsidiary or angular momentum quantum number, l, which specifies the shapes of the orbitals.
The magnetic quantum number, m, which specifies the orbital orientation in space.
The spin quantum number, s, which specifies the direction in which the electron is spinning. These quantum numbers completely describe the stationary stage of the electron.
Example
(a) Distinguish between the following terms:-
(i) An orbital and orbit.
(ii) S – Orbital and P – orbital.
(iii) A quantum of light and quantization of energy.
(iv) Quantum shell and quantum number.
(b) Explain briefly the meaning of the following quantum numbers:-
(i) n
(ii) l
(iii) m
(iv) ms
(c) In a tabular form specify all the four quantum number for each electron in an atom whose n value is 2. Given all the orbitals are full of electrons.
(d) Given the value of the quantum numbers n, l and m for the electron with the highest energy in sodium atom in the ground state.
(e) Write down all the quantum number of all the electrons in the ground state of nitrogen atom.
(f) Give the values of all the four quantum number for 2p electrons in Nitrogen.
(g) Briefly explain why the following quantum numbers are not allowed.
i) n = 1, l = 1, m = 0
ii) n = 1, l = 0, m = 2
iii) n = 4, l = 3, m = 4
iv) n = 0, l = -1, m = 1
v) n = 2, l = -1, m = 1
Solution
(a) (i) An orbit is a well define circular path in which electrons were assumed to revolve around the nucleus. Whereas an orbital is a three dimensional region of space around the nucleus whereby there is high probability of finding an electron.
(ii) S–Orbital is spherical and hence non directional whereas p-orbital is dumbbell in shape and it is directional.
(iii) A quantum of light is a photon of radiation emitted when an electron jumps from one energy level to another whereas quantization of energy means energy emitted or absorbed following electron transition between one energy level and another is not continuous rather it is in form of small packet called quanta.
(iv) A quantum shell is the energy level of an electron in a given atom whereas quantum number refers to specifies way of defining an electron in a given atom whereas quantum number refers to specified way of defining an electron in a given orbital of an atom using n, l, m and ms values.
(b) (i) n – represents the principle quantum number;
It specifies the location and the energy of an electron.
It is a measure of the volume of the electrons cloud.
As the value of n increases or becomes less negative it means the energy levels of the electrons get further away from the nucleus n can have only integers 1, 2, 3, 4 to infinite represented by K,L,M,N etc. Also called azimuthal quantum number or subsidiary quantum number, specifies the shapes of the orbital, when n = 1 there’s only l s orbital where n = 2, there’s 2s and 2p orbital, thus for a given value of n, l, are all from 0, 1, 2…up to l = n – 1. Thus when n = 4 values are 0, 1, 2 and 3. Which represents 4s, 4p, 4d and 4, â”” sub–levels m  Magnetic quantum number specifies the number of orbital present in a given value of â””.
Magnetic quantum number specifies the number of orbital present in a given value of â””.
It specifies the orientating i.e. the direction of the orbital to magnetic field in which it’s placed.
It accounts for the splitting of the spectral lines observed when an atom emitting radiation is placed in a magnetic field, ms – Refers to spin quantum number.
(i) It indicates the direction in which the electron spins.
(ii) Only two values are allowed for an electrons i.e. electrons may spin clockwise, shown as +1/2 or 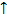 may spin anticlockwise as shown as ½ or
may spin anticlockwise as shown as ½ or 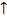
(c)
|
Principle (n) |
Azimuthal |
Magnetic quantum |
Spins ms |
|
n = 1 |
â”” = 0 |
m = 0 |
+1/2 , –1/2 |
|
n = 2 |
â”” = 0 â”” = 1 |
m = 0 m = -1 m = 0 m = 1 |
+1/2 , –1/2 +1/2 , –1/2 +1/2 , –1/2 +1/2 , –1/2 Total of 10 electrons |
edu.uptymez.com
(d) When n = 3 in Na atom that electron is found in s – orbital thus â”” = 0 and also ml = 0
(e) 1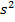 electrons:
electrons:
(1st) n = 1, l = 0, m = 0, s = +1/2 (2nd) n = 1, l = 0, m = 0, s = -1/2
Electrons:
1st n = 1, l = 0, m = 0, s = +1/2 (2nd) n = 1, l = 0, m = 0, s = -1/2
2 Px; n = 2, l = 1, m = +1, s = +1/2 2Py; n = 2, l = –1, m = –1, s = +1/2
2Pz; n = 2, l = 1, m = 0, s = +1/2
(f) 2P electrons, there’s
2Px; n = 2, l = +1, m = +1, s = +1/2 2Py: n = 2 , l= –1, m = –1, s = +1/2
2Pz: n = 2 l = 1 m = 0, s = +1/2
(g) (i) l value must be smaller than n value since l = n-1
(ii) When l = 0 value of m is only 0
(iii) For l= 3, m can range from –3 to +3. Thus m=+4 is not allowed
(iv) n value cannot be equal to zero.
(v) l value cannot be a negative number.
Example
(a) briefly explain the meaning of the following quantum numbers:-
(i) ml
(ii) n
(iii) l
(iv) ms
(b) If n = 2, tabulate the values of 1, ml and ms.
(c) Give the possible values of 1 and m, for an electron with the principal quantum number, n= 3
(d) Briefly explain why an electron cannot have the quantum number n = 2, l = 2 and ml = 2
Solution
(a) (i) ml – Magnetic quantum number with values from -l to +l. These are numbers showing the number of sub – level in each energy level.
– Describes orientation
(ii) n – Principal (shell) Quantum number value of 1, 2, 3 etc theses are numbers used to represent the main energy level in which an electrons is found.
(iii) l – Azimuthal or subsidiary or Angular or orbital Quantum number. They specify the shape of orbitals.
(iv) ms – Magnetic spin Quantum number with values +1/2 and –1/2 shows the spinning of electrons in orbitals (clockwise and anticlockwise direction).
(a) For n = 2, the scheme below illustrates the value of 1, ml and ms.
l=0, 1
ml=-1, 0, +1, 0
ms= ±1/2, ±1/2, ±1/2, ±1/2
Therefore the total number of electrons = 8
(b) If n = 3, possible values of 1 and m are;
l = 0, 1 and 2
m = 0 for l = 0
m = –1, 0 and +1 for l = 1
m = –2, –1, 0, +1 and 2 for l = 2
Note that: l=n-1
(c) An electrons cannot have quantum numbers, n = 2, l = 2 and m = 2. This is because when n = 2, l can be 0 and 1; then ml can be 0, –1, 0, +1 different values which can occupy in orbitals (i.e. they have n, l and m values the same but they must spin in opposite directions and hence have different ms values).