SOLUTIONS
What is a solution homogeneous?
A solution is a uniform mixture of two or more substances.
The substance which are mixed to form solution are also termed as components
Example of solution
The solution formed can be
i) Solution of liquid in liquid
ii) Solution of solid in liquid
iii) Solution of gas in liquid
iv) Solution of gas in gas
SOLUTION OF LIQUID IN LIQUID
This is the solution formed when two or more liquids are mixed to form a uniform homogeneous mixture.
When the solution of liquid in liquid is formed the liquids are to be miscible.
When the solution is formed the saturated vapour pressure depends on the composition of the components.
The composition of the components is also depends on the mole fraction of such component.
Example
What is mole fraction
Mole fraction Is the ratio of number of a liquid to the total number of moles of all liquid present in the container.
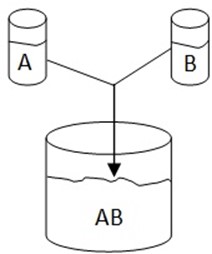
Consider the solution formed by mixing liquid A and B
Then
Let : nA be number of moles of liquid A
nB be number of moles of liquid B
Total number of moles = nA + nB
nT = nA + nB
Then to get mole fraction:
ΧA = mole fraction of A
XB = mole fraction of B
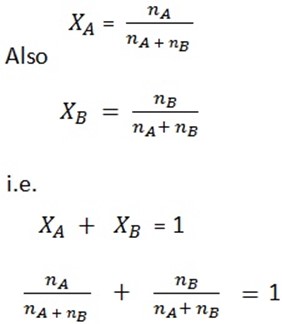
When expressed in decimal
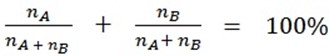
When expressed in percentage
The mole fraction can also be calculated interns of partial pressure ie If the liquid to be mixed are A and B
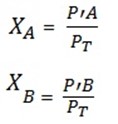
RAOULT’S LAW OF PARTIAL PRESSURE
The Raoult’s law of partial pressure states that
“The saturated vapour pressure of each component in a mixture is equal to the product of mole fraction of that component and its pure vapour pressure”
ASSUMPTIONS OF RAOULT’S LAW
For the Raoult’s law to be feasible the following assumptions are to be considered.
i) Intermolecular forces of attraction should be equal to the intermolecular forces of attraction.
ii) After mixing the component there must be no change in volume.
iii) There must be No change in temperature.
iv) The liquids must be miscible.
v) The liquid should not react.
CONCLUSION
The solutions that do obey all assumption of Raoult’s law are called IDEAL SOLUTION. While the liquid which deviate from the assumption of Raoult’s law are termed as NON – IDEAL SOLUTION/REAL SOLUTION.
IDEAL SOLUTION
In an Ideal solution the cohesive forces between its molecules. In an ideal or perfect solution, the cohesive forces would be just the same as those existing in the separate components of the solution. A solution made from A and B would only be ideal if the forces existing in the solutions of A and B were just the same as those existing in pure A and pure B.
Ideal solutions are rare, but they are most likely same to occur with mixtures of two almost identical chemicals e.g. hexane and heptane. Most solutions deviate considerably from the ideal because the interactions within the solution are different from those in the pure liquids.
Ideal solution depends on:
1) Vapour pressure of Ideal solution
2) Boiling point of Ideal solution