AROMATIC COMPAOUNDS (ARENES)
These are organic compounds with benzene ring as functional group.
Molecular formula of benzene is C6 H6.
-It is highly unsaturated molecule but it does not undergo reaction readily and it tends to undergo substitution reaction.
STRUCTURES OF BENZENE
Structure of benzene can be expressed (shown) by using;
i. Kekule structure
ii. Resonance structure
I. KEKULE STRUCTURE (1865)
According to kekule;
-Structure of benzene is hexagonal ( It is cylic structure with six carbon atoms).
-In structure of benenze carbon-carbon double bond alternate carbon – carbon single bond.
-The structure of benzene is interconvertable.

STRENGTH OF KEKULE STUCTURE
-It gives correct molecular formula of benzene which is C6H6.
-It is true that C-H bond in benzene are all alike. (This can be seen though x-ray diffraction).
WEAKNESS OF KEKULE STRUCTURE
-It fails to explain why benzene does not undergo addition reaction readily and it tends to undergo substitution reaction in steady.
-Through x-ray diffraction it can be seen that carbon – carbon bond are equal throughout the benzene the fact which can not be explained to by Kekule structure (According to kekule structure there is C=C and C-C so it was expected that bond length of c=c to be shorter than that of c-c).
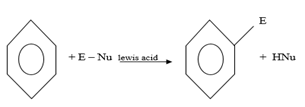
EXAMPLES OF ELECTROPHILIC SUBSTITUTION REACTIONS IN BENZENE
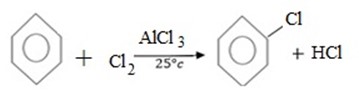
a) (a) HALOGENATION
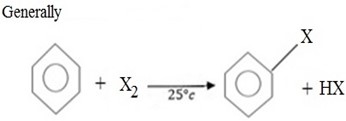
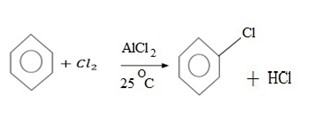
MECHANISM
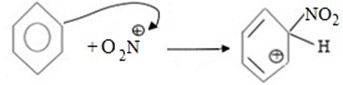
i. Formation of an electrophile.
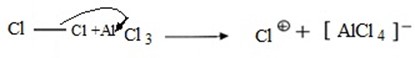
ii. Formation of intermediate carbonium ion.
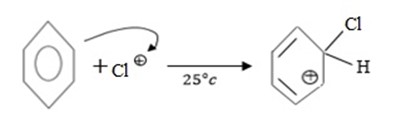
iii. Formation of product and regeneration of catalyst.
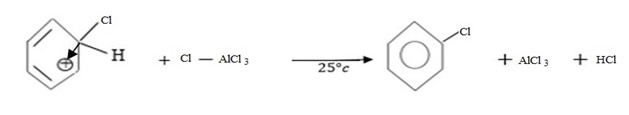
Thus, Overall reaction is
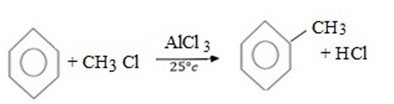
(b) ALKYLATION (FRIDEL CRAFT ALKYLATION)
Craft alkylation is the electrophilic substitution reaction between Benzene and haloalkane under presence of lewis acid catalyst to give alkylbenzene.
Generally;
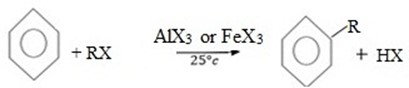
Example.
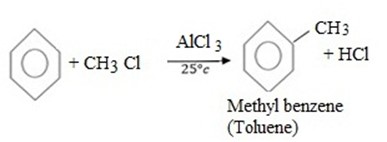
MECHANISM
- i Formation of an electrophile.
edu.uptymez.com
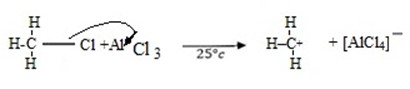
ii. Formation of intermediate carbonium ion.
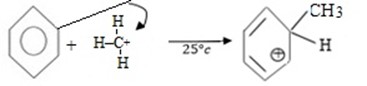
iii. Formation of product and regeneration of catalyst.
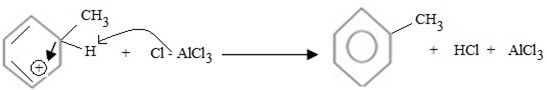
Hence, overall reaction.
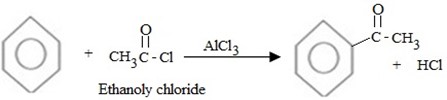
(c) ACYLATION (FRIDEL CRAFT ACYLATION)
Fridel crafit acylation is the electrophilic substitution reaction between benzene and acyl compounds under presence of lewis acid catalyst aromatic ketone.
Generally;
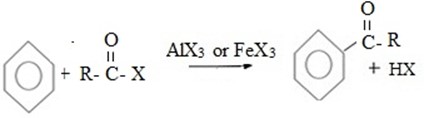
MECHANISM
i.Formation of an electrophile.

ii. Formation of intermediate carbonium ion.
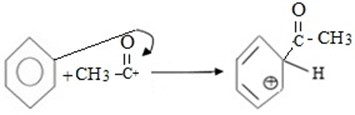
iii. Formation of product and regeneration of catalyst.
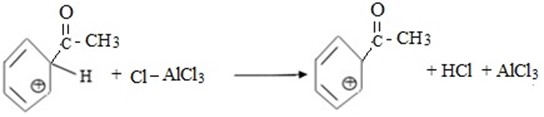
iv.
Thus, overall reaction is
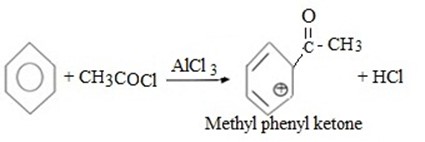
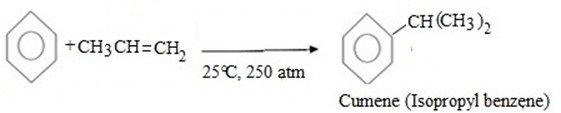
(d) CUMENE FORMATION
Bnzene react with propene under presence of acid medium to give isopropyl benzene (cumene)
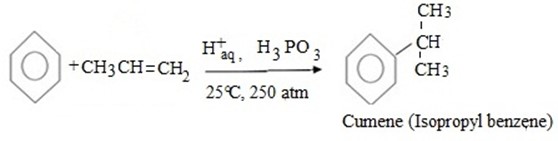
MECHANISM
i.Formation of an electrophile.
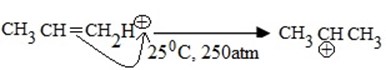
ii. Formation of intermediate carbonium ion.
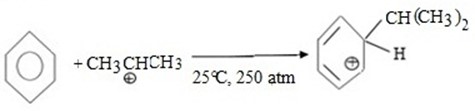
iii. Formations of product and regeneration of catalyst.
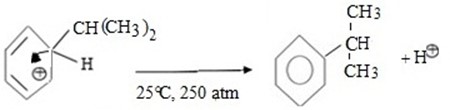
Thus, overall reaction is
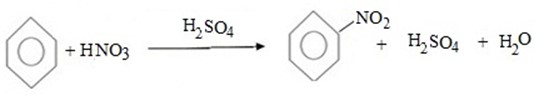
(e) NITRATION
Benzene react with Nitric acid under presence of sulphuric acid yielding nitrobenzene.
i.e
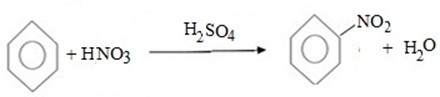
MECHANISM
i. Formation of an electrophile.
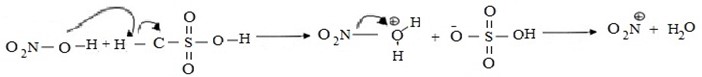
ii. Formation of intermediate carbonium ion.
iii. Formation of product and generation of catalyst.
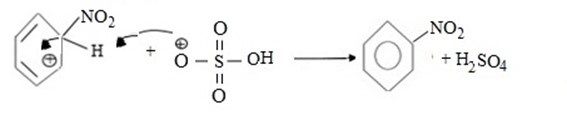
Hence, overall reaction is
Benzene react with sulphur trioxide (or concentrated sulphuric acid) to give sulphobenzene (Benzene sulphoric acid).
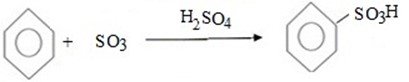
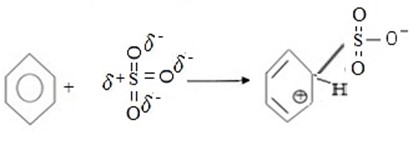
MECHANISM
i. Formation of an electrophile.

ii. Formation of intermediate carbonium ion.
iii. Formation of product.
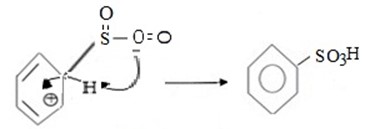
Thus, overall reaction is
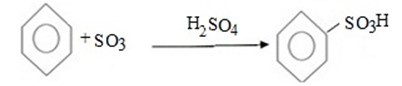
Also.
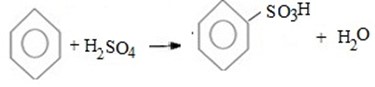
Above reaction sulphuric acid Itself is good lewis acid (There is need of another lewis acid catalyst).
DIRECT EFFECT IN MONOSUBSTITUETED BENZENE
ACTIVATOR AND DEACTIVATOR
- Reactivity of benzene towards electrophile (in eletrophilic substitution reaction of benzene) depend on the electrons density in benzene ring.
- If the electron density is high then benzene will be more reactive towards electrophile and if it is low than the benzene will be less reactive toward an electrophile.
- When substitutients in benzene increase electron density in benzene ring, then the substituents in said to increase reactivity of benzene towards an electrophile.
- So any factor which affect the electron density in benzene ring is said to affect reactivity of benzene towards an electrophile.
- When substituents in benzene increase electron density in benzene ring, Then the substituents is said to increase reactivity of benzene towards an electrophile. i.e it is said to activate electrophilic substitution reaction of benzene and hence the substituent is known a ACTIVATOR.
edu.uptymez.com
· On other hand if the substituents decrease electron density in benzene ring, then the substituents is said to decrease reactivity of benzene towards an electrophile. i.e It said to deactivate electrophilic substitution reaction of benzene and hence the substituent is known as DEACTIVATOR.
Qn. How we can recognize the substituents is activator or Deactivator?
ANS
Before studying recognisation of activators and deactivators. It is better to study first effect which cause activation and deactivation in benzene.
There are two effect which cause activation in benzene.
i. Positive Inductive effect (+I).
ii. Positive mesomeric effect (+M).
i. POSITIVE INDUCTIVE EFFECT (+I)
This is the effect which arise in the organic compounds as a result of partial movement of electron pair towards the functional group. (In this case benzene ring).
ii. POSITIVE MESOMERIC EFFECT (+M)
This is the effect which arise in the organic compounds as a result of total movement of an electron pair towards the functional group ( in case benzene ring) and move back again to its original position within the same molecule. Thus +M to activation in benzene substituents which cause +M (in benzene) are those with atoms possessing pair or negatively charged atom and It self directly bonded to another atom by sigma (δ)bond.
Example
OH–, NH2–, RO–, X.
Other hand there are two effects which cause deactivation in benzene.
i. Negative Inductive effect (-I).
ii. Negative mesomeric effect (-M).
i. NEGATIVE INDUCTIVE EFFECT (–I)
This is the effect which arise in organic compound as result of partial withdraw of an electron pair from functional group. (in this case benzene ring).
Inductive effect do deactivate of the benzene by partial withdraw of electron pair from benzene ring.
Substituent which cause (-I) are strong electronegative atom or electron attracting radicals.
Examples. OH–, X, etc.
ii. NEGATIVE MESOMERIC EFFECT (-M)
This is the effect which arise in organic compounds as a results of partial withdraw of an electron pair from functional group (in this case benzene ring) and then moving back again to the original position within the same molecule.
- So -M do deactivation in benzene by withdraw of an electron pair from benzene ring.
- Substituents which cause –M are those with atoms possessing pair or negatively charged electron and itself is bonded to atom by π-bond.
edu.uptymez.com
Example:
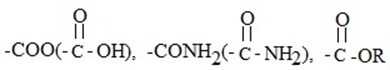
· There is the case where there is competition between mesomeric effect and Inductive effect. i.e the same substituent cause negative inductive and positive mesomeric effect (+M).
· When this occur in most cases mesomeric effects tends to outweighs Inductive effects i.e when the same species cause –I and then the effect at which will be considered is +M and these will be ACTIVATOR (Not deactivator).
- Halogens are exceptional of above explanations i.e In halogens Inductive effects tends to outweighs mesomeric effects why?
edu.uptymez.com
REASONS
- Halogens are strongest electronegative element among all substituent of benzene as result of their smallest atomic size. This make halogens to exert strongest negative inductive effect.
- On other hand Halogens have maximum number of lone pair electron, thus making less available in participation of mesomerism thus make Halogens to exert weakest mesomeric effect among all substituents.
- So while Halogens exert strongest negative Inductive effect it also weakest effect (-M) hence in halogens Inductive effect weighs mesomeric effect.
- Generally we can conclude that all substituents which cause positive inductive effect and those which cause positive mesomeric exceptional of halogens are ACTIVATOR. And all substituents which cause negative mesomeric effect with addition of Halogens (which –I ) are DEACTIVATOR.