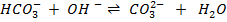Arrhenius concept of acids and bases .
What is an acid? (according to Arrhenius concept of acids and bases)
Ø Arrhenius considered that an acid is a substance which when dissolved in water dissociate to produce H+ ions as the only positively charged ions i.e.
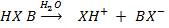
e.g
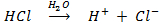
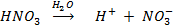
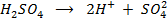
Ø -He considered a base to be a substance which produce hydroxyl ions when dissolved in water as the only negatively charge ions i.e
e.g.
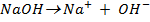
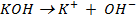
The neutralization of acid with a base yields to a salt and water.
e.g.
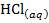 +
+ 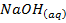
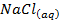 +
+ 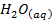
Ionic equation
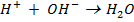
According to Arrhenius, neutralization reaction is all about formation of water.
Weakness of Arrhenius equation
1.
i) This concept is limited to water. It refers to H+ and OH– ions derived from water. A true general concept of acid and base should be appropriate to other solvent like liquid NH3 and alcohols.
2. ii) The concept does not provide the room for acids and bases which do not contain H+ ions and HO – ions.
Bronsted – Lowry concept of acids and bases
–
Bronsted and Lowry proposed a theory of acids and bases applicable to all solvents.
– They proposed that, an acid is any substance that can donate a proton to any other substance.
e.g
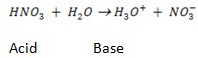
– A base is a substance that can accept a proton from any other substance.
E.g. 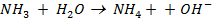
Base Acid
– – They are called a monoprotic acid an acid which donates only one proton.
e.g. HNO3, HCl
– – Diprotic acid can donate two protons.
E.g. H2SO4
– – A polyprotic acid is an acid that can donate more than one proton.
E.g. H2SO4, H3PO3, H2C2O4
– A polyprotic base is one which can accept more than one proton.
E.g. 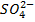
– – A monoprotic base is one which can accept only one proton.
E.g.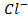 ,
,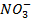
NOTE: HCl and Cl– are acid-base conjugate pair. Another example is HNO3 and 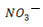
Amphoteric (amphiprotic) acids and bases.
These behave as bronsted – Lowry acids or bases
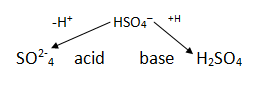
Conjugate acid base pair
-For every acid, there is a corresponding (conjugate) base to accept a proton
E.g. 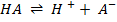
Acid base
(Proton donor) (Proton acceptor)
HA and A– are conjugate pair i.e.
HA is a conjugate acid of A– and A– is a conjugate base of HA.
® In a solution, there must be a base to accept a proton
E.g. 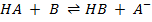
Acid Base Acid Base
E.g. 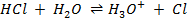 –
–
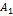
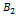
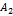
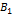
A1, B1, and A2, B2, are acid base conjugate.
NOTE:
From Bronsted–Lowry concept of acid and bases, the stronger the acid, the weaker it’s conjugate base and the stronger the base, the weaker its conjugate acid.
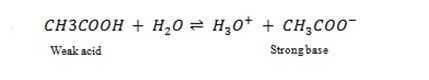
–
CH3COOH is a weak acid, but its conjugate base i.e. 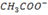 is a strong base.
is a strong base.
– H2O is a weak base, but 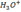 is strong acid.
is strong acid.
Advantage of Bronsted–Lowry concept over Arrhenius
® It can apply to any solvent not necessarily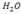 . Here the definition of bases is much wider.
. Here the definition of bases is much wider.
Weakness:
Since the concept is based on proton transfer, it does not consider other compounds which do not contain hydrogen i.e AlCl3, BF3, SO3.
In contrast to Arrhenius theory, acid and bases are no longer related to salts (by neutralization).
Question 1:
A. Define
i) Conjugate acid-bases pair.
ii) Conjugate base.
B. For the following pairs, write down the equation to show the conjugate acid-bases pair.
i) 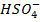 /
/ 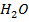
ii) 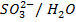
iii) 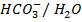
iv) 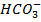 /
/ 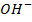
Question 2:
a. Write the formula and give the name of the conjugate base for each of the following acids.
i) 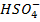
ii) 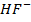
b. Write the name and formula of the conjugate acids for each of the following bases.
i) NH3
ii) Br –
iii) HS–
Question3:
In each of the following acid base reaction. Identify the acid and the base on the left and their conjugate partners on the right.
base reaction. Identify the acid and the base on the left and their conjugate partners on the right.
a) 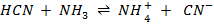
b) 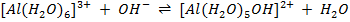
ANSWERS:
a) 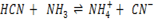
Acid Base Acid Base
HCN, CN– is a conjugate pair and NH3, NH4+ is a conjugate pair
b)
HSO–4, SO4-2, is a conjugate pair
c) 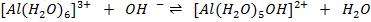
Acid Base Base Acid
[Al(H2O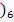 ] 3+, [Al(H2O) 5 OH] 2+ is a conjugate pair.
] 3+, [Al(H2O) 5 OH] 2+ is a conjugate pair.
OH– and 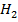 O is a conjugate pair.
O is a conjugate pair.
Q: Conjugate acid-base pair is a pair which shows that for every proton lost by acid, there is a corresponding base to accept it.
a) Conjugate base.
b)
i) 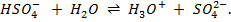
ii) 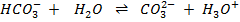
iii)