i) STRUCTURES OF AMINES
Amines are derivatives of ammonia in which one or more hydrogen have been replaced by alkyl group or aryl group.
General formula:

If two hydrogens have been replaced we have;
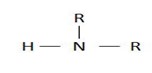
2° amine
If 3 hydrogens have been replaced we have;
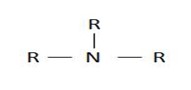
3° amine
NOTE:
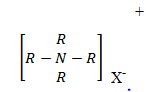
Quatenary salt of amine
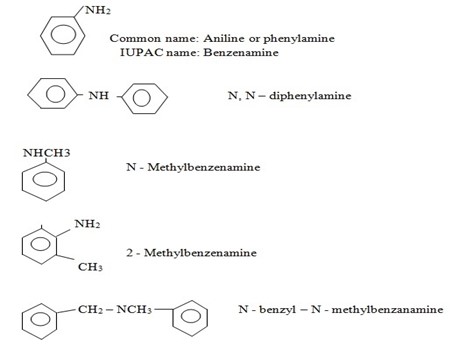
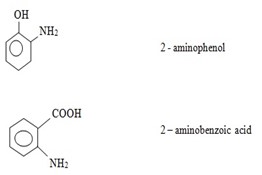
ii) (NOMENCLATURE) IUPAC SYSTEM
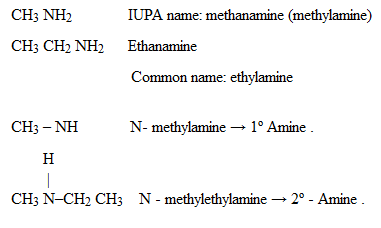
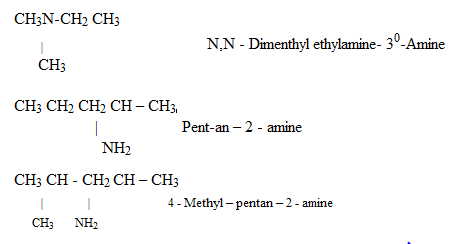
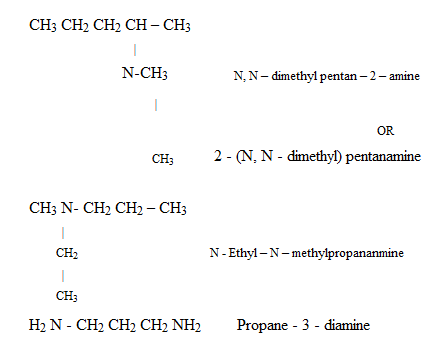
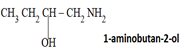
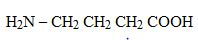 4 – aminobutanoic acid
4 – aminobutanoic acid
iii ) PREPARATION OF AMINES
1.ALKYLATION OF AMMONIA WITH HALOALKANES
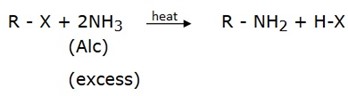
1 amine can be obtained by reaction of alkyl halide with excess ammonia.
amine can be obtained by reaction of alkyl halide with excess ammonia.
Why excess ammonia?
So as to prevent the formation of 20 amine or 30 amine. Therefore, this is not a suitable for preparation of amine because the substitution of hydrogen does not stop at the first stage.
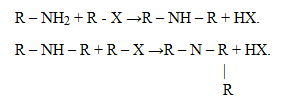
2. REDUCTION OF NITROALKANES (R – NO2)
10 amines can be obtained by reduction of nitroalkane. The reducing agent is LiAlH4 or Sn/HCl or Fe/HCl
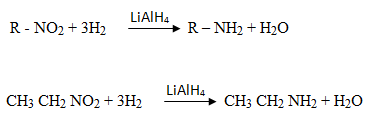
3. REACTION OF AMIDES WITH LiALH4
1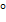 amides can be reduced to 10 amines
amides can be reduced to 10 amines
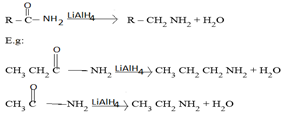
4.AMMONIATION OF CARBONYL COMPOUND FOLLOWED BY REDUCTION WITH HYDROGEN IN THE PRESENCE OF NICKEL
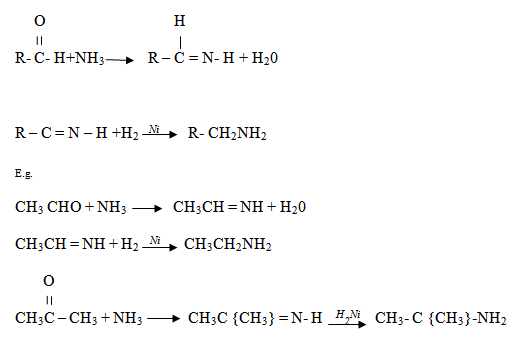
5. REDUCTION OF NITRILES
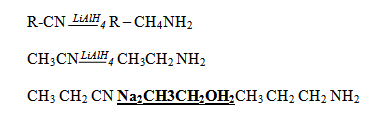
6. REACTION OF ALCOHOLS WITH AMMONIA
Catalytic ammoniation of alcohol gives a mixture of amines.
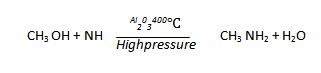
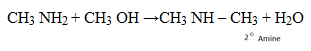
7. HOFFMAN’S DEGRADATION OF AMIDES
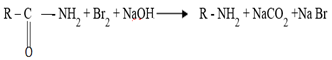
It is important reaction in the conversion of amide to amine with one carbon less.
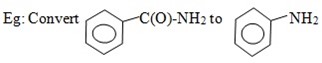
(i) By Hoffman’s degradation reaction
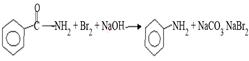
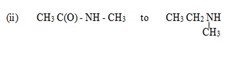

Solution:
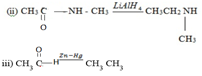
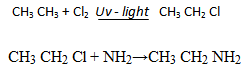
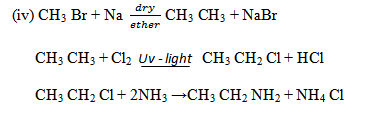
4.PROPERTIES OF AMINES
a)PHYSICAL PROPERTIES OF AMINES
Boiling point and melting point
Amines have high boiling and melting points due to their ability to form hydrogen bonding as compared to hydrocarbons.
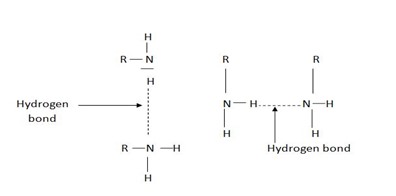
(i) Its boiling point is lower than alcohol (R – OH)
Reason: In oxygen, there are 2 lone pairs which increase its chance of hydrogen bonding.
It also has lower boiling point than carboxylic acid.
10 amines have high boiling point than 20 amines. For 30 amines, there is no possibility of H –bonding since Nitrogen is not attached to hydrogen.
(ii) Solubility:
Lower aliphatic amines are soluble in water.
Reason:- They can form hydrogen bond with water.
As the molecular mass increases, solubility decreases.
Reason: -The long chain of hydrocarbon (R) is insoluble since it is water phobic. As the alkyl group becomes bigger, solubility decreases.
Amines containing 6 or more carbon do not dissolve in water.
E.g: aniline is insoluble due to this reason.
b)CHEMICAL PROPERTIES OF AMINES
1. Basic character
Amines are the most important organic bases. Aliphatic amines are slightly stronger Lewis bases than ammonia. Lone pair an the nitrogen is readily available to accept proton in amines than it is an ammonia .
R R— Positive inductive effect
| |
R-NH2 R-N-H R-N-R
20 amine the strongest base among the three
The stability of hydrated ion decrease from 10 amine 30 amine
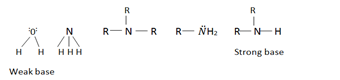
Reason:- 30 amines forms a very unstable ammonia salt. Although it can easily donate a lone pair of electrons as compared to 10 and 20 amines, it forms very unstable hydrated ion.
Note:
The inductive effect on alkyl group tends to make 30 amines more basic while hydration effect tends to make 1 amine more basic. As a result of the combined effect of these two the observed order of the base strength will be;
amine more basic. As a result of the combined effect of these two the observed order of the base strength will be;
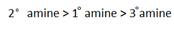
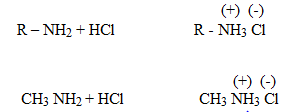
Methylammonium chloride
Q. Between the following amines, which is stronger base
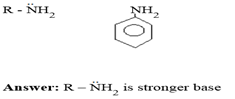
Reason:- In Aniline there is negative mesomeric effect since lone pair is withdrawn from the amine group.
Mesomerism of aniline
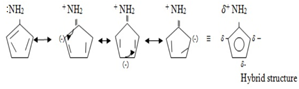
For aromatic compounds, the lone pair is less readily available for protonation (addition of proton)
EFFECT OF SUBSTITUENT GROUP ON THE BASICITY OF ANILINE
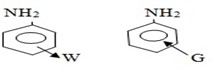
· All activators make aniline more basic while deactivators make aniline less basic.
Electrons supplying groups
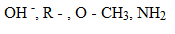
Electrons withdrawing groups
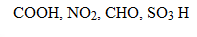
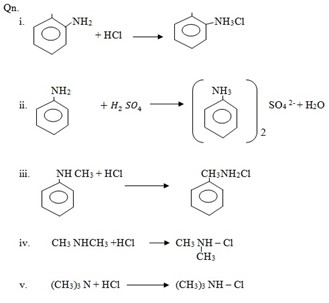
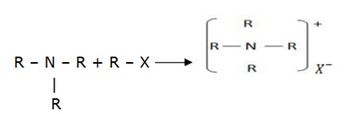
2. Alkylation (reaction with haloalkanes)
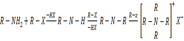

Example:
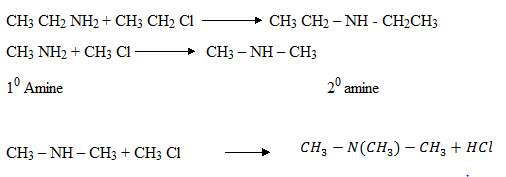
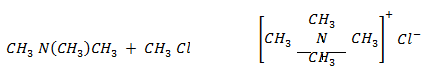
Quatenary salt
3. Reaction with ketones and aldehydes
Both aliphatic and aromatic 10 amines react with aldehydes and ketones to form a base known as Schiff’s bases.
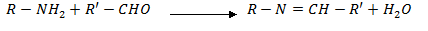 Schiff’s base
Schiff’s base
E.g:
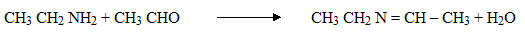
-Lewis acid is a substance that accepts a lone pair of electrons
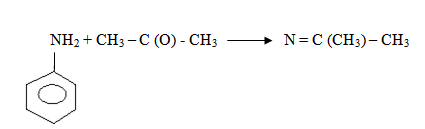
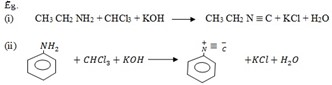
4. Reaction of 10 amines with chloroform (carbylamines reaction)
· Both aliphatic and aromatic amines on heating with chloroform and alcoholic KOH given isocyanides (carbylamines)
· The isocyanide form has offense smell. It is used to identification test for 10 amine
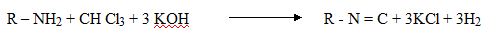
Carbylamines (offense smell)
This reaction is a Test for 1 amine
amine
(i) Convert;
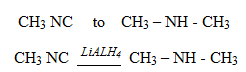
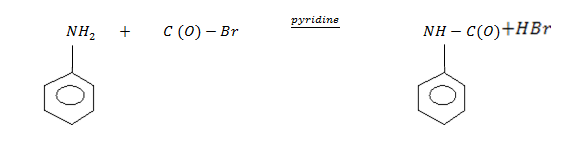
5 . Acylation
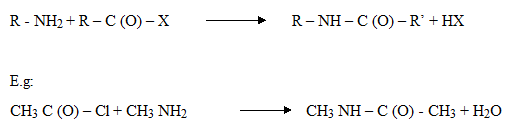
N – methylethanamide
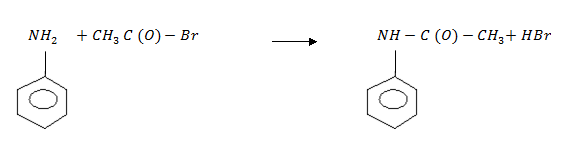
N – phenylethanamide
6. Reaction with Nitrous acid 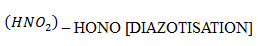
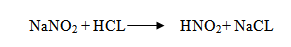
Note: It is used as a distinguishing test between different classes of amines.
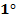 Amine
Amine
· Aliphatic 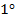 amine reacts with HONO to form alcohol and nitrogen gas (you will see bubbles)
amine reacts with HONO to form alcohol and nitrogen gas (you will see bubbles)
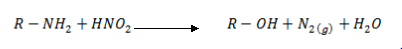
E.g:
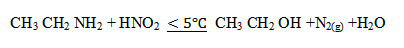
– For aromatic amine react with HNO2 in cold (below 5ºC) to form diazonium salt.
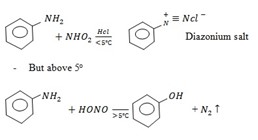
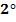 Amine
Amine
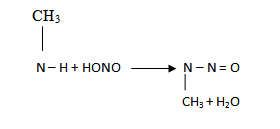
·  amines react with nitrous acid to give N – nitrosoamine which separate out as a yellow oily liquid.
amines react with nitrous acid to give N – nitrosoamine which separate out as a yellow oily liquid.

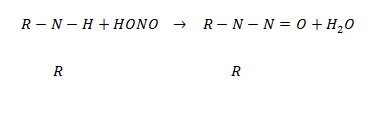
N- nitrosoamine
(Yellow oil liquid)
It is a distinguishing test since we see yellow oil liquid
Eg:
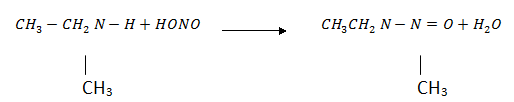
 Amine
Amine
 Amine reacts with nitrous acid to form a soluble trialkylammonium salt which is colourless.
Amine reacts with nitrous acid to form a soluble trialkylammonium salt which is colourless.
It is not a distinguishing test since there is no observable change.
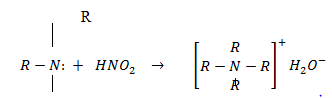
Aromatic 3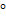 amines react with HONO to give green colour p – nitrosoamine
amines react with HONO to give green colour p – nitrosoamine
This is a distinguishing Test since there is a green colored
Reaction of amines with metal ions Ag+ & Cu2+
Reaction of aromatic amine due to benzene ring
1. reaction with bromine water
· Aniline undergoes halogenations even in the absence of catalyst. With bromine it gives
2, 4, 6 – tribromoaniline (white solid)
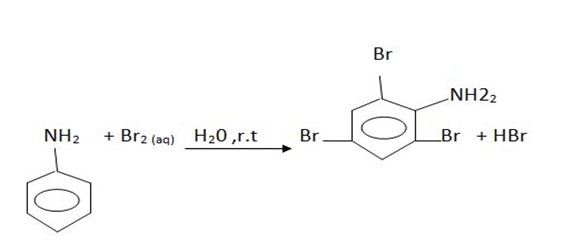
Here benzene ring is highly activated due to the presence of amine
To obtain mono substituted derivative, aniline should be first acylated then halogenation. After halogenations, the acyl group is removed by hydrolysis to obtain mono substituted aniline.
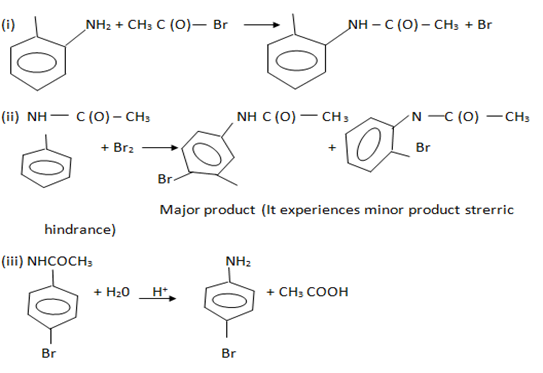
2. Nitration
Direct Nitration is not possible because aniline gets oxidized. Amine group should be protected by acylation, then nitration and finally hydrolysis.
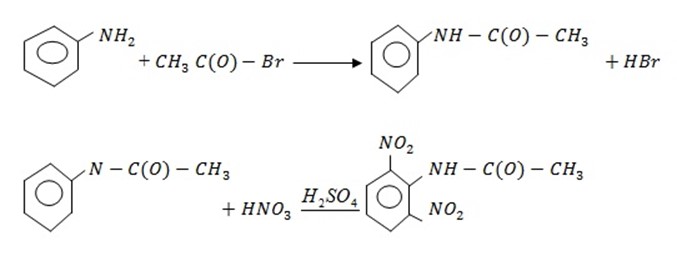
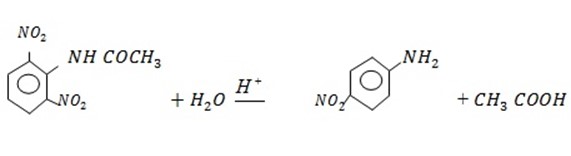
3. Sulphonation
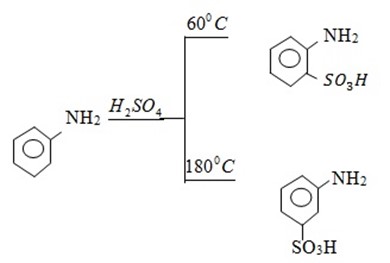
We have different products due to H – bonding. At 1800C, H – bonding will not be effective hence SO3H will be at para position.
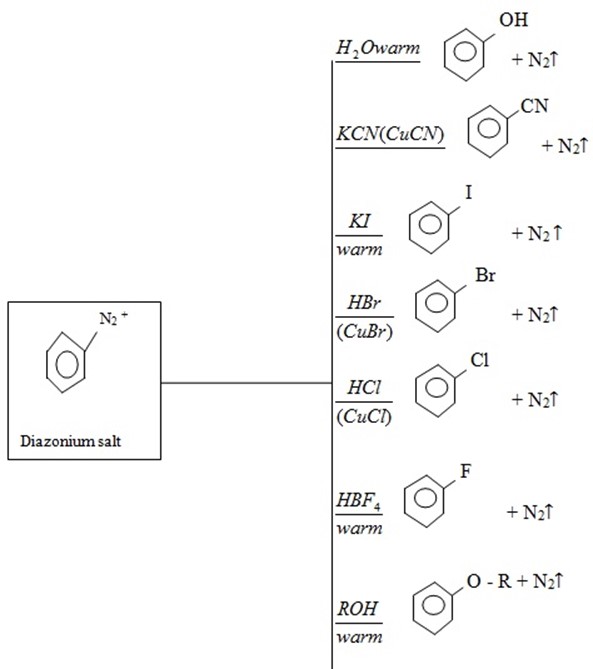
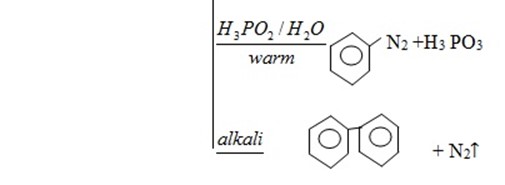
4. Diazonium salt
· It is used to form many other products.
· Reaction is nucleophilic substitution  .
.