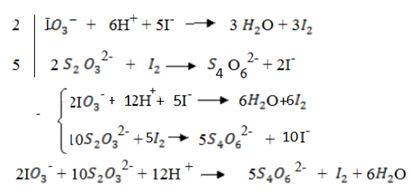1 . Explain very briefly using equations where possible the extraction of copper from its commercial hall under the heading of reduction and
purification.
2. (a) Write down:
(i) Four reasons which justifies the placement of hydrogen in group I of the periodic table
(ii) Four reasons which puts hydrogen in group (vii) of periodic table
(b) (i) In which group do you think hydrogen should strictly belong
(ii) Give reasons for your answer for (i) above
(c) Describe the action of water on hydrides of period III
(d) Compare the thermal stability of carbonates of group I and II by using a specific example show their differences.
3. Comment with help of chemical equations where necessary in the following:
(i) Iron (ii) chloride cannot be prepare by heating Iron filing in a steam of chlorine gas.
(ii) Hydrochloric acid cannot be used as acidic medium during redox titration of KMnO4 against Fe SO4.
(iii) Solid Al (OH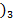 is soluble in aqueous solution of NaOH.
is soluble in aqueous solution of NaOH.
ANSWER:
1. Extraction of copper
Steps:
Roasting:
Cu Fe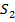 is heated in plenty supply of air
is heated in plenty supply of air
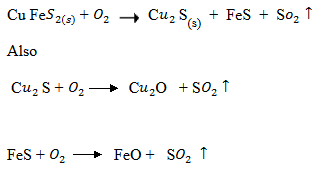
Now silica is added to Fe to remove FeO
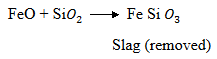
Self – Reduction reaction:
In self-reduction reaction, C S reacts with C
S reacts with C O to form molter copper which later solidifies
O to form molter copper which later solidifies
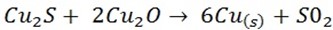
Purification:
Purification is done electrolytically.
At cathode
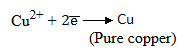
At anode
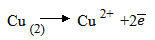
(c) Solution.
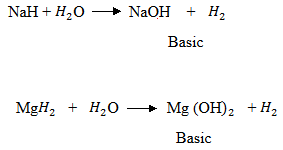
The hydrides of Na and Mg form basic solution.
The hydride of sulphur – chlorine form acidic solutions due to presence of hydroxonium ion.
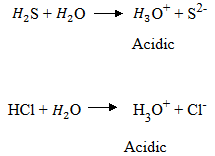
The hydrides of phosphorus i.e. phosphine is amphoteric
(d) Group I:
Carbonates of group I are thermally stable due to the large size of the atoms hence it will be capable of accommodating the carbonate ion.
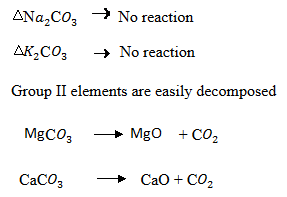
3. (i) Chlorine is a strong oxidizing agent hence it will oxidize iron straight to Iron III chloride
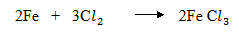
(ii) Cl– may react with Fe3+ (titration product) which will form FeC which is red in colour.
which is red in colour.
This will make change in colour of MnO4 be difficult to account for (or difficult to notice)
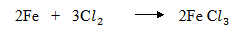
1.
4. (a) Define the following
(i) Atomic radius
(ii) Ionization energy
(b) Contrast the action of heat on the following pairs of compounds
(i) NaN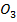 and Ca (N
and Ca (N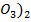
(ii) N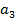 C
C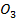 and PbC
and PbC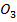
(c) Describe how you can distinguish chemically the following pairs of compounds
(i) Mg (OH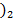 and Mg C
and Mg C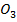
(ii) NaCl and Al C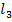
(iii) Cu S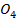 and Cu (N
and Cu (N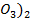
2. 5. (a) Explain the following by chemical equation. where necessary:
Solid carbonate of iron (iii) and Al have never been isolated
(i) Lime water is used to test the presence of Co2 gas
(ii) When sodium hydrogen carbonate is added to copper (ii) sulphate effervescence is observed.
(iii) Lead hydroxide ppt dissolves in excess sodium hydroxide solution.
(b) By using equation. Where necessary describe:
(i)Two methods of preparing copper (ii) chloride in the lab.
(ii) Describe how ZnO reacts with both acid & bases.
(c) List 2 important uses of CaO in daily life
6. (a) Define
(i) Heat of reaction
(ii) Bond energy
(b) The enthalpy of formation of CC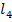 is – 135.5 KJmol– and enthalpyies of atomization of graphite and chloride are 715 and 121.2KJ respectively. Calculate the C-Cl bond energy
is – 135.5 KJmol– and enthalpyies of atomization of graphite and chloride are 715 and 121.2KJ respectively. Calculate the C-Cl bond energy
(c) (i) When 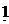 g of C, H2 and C
g of C, H2 and C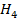 are completely burnt in excess oxygen 32, 143 and 56 KJ of heat is liberated. Calculate the standard enthalpy of formation of C
are completely burnt in excess oxygen 32, 143 and 56 KJ of heat is liberated. Calculate the standard enthalpy of formation of C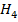
(ii) The heat of formation of Fe2 O3 is – 824.OKJml-1. What will DH of the reaction

ANSWERS:
4. (a) (i) Atomic radius is half the internuclear distance between two similar atoms covalently bonded by a single bond
(ii) Ionization energy is the energy required to remove the most loosely held election from the shell of an atom.
(b) (i) NaN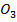 is more stable than Ca (N
is more stable than Ca (N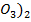 because sodium is highly electropositive hence bond energy between sodium and nitration is high hence it comes very stable.
because sodium is highly electropositive hence bond energy between sodium and nitration is high hence it comes very stable.
(ii) N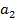 C
C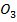 is more stable than PbC
is more stable than PbC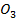 since sodium is highly electropositive hence there is high bond energy between sodium and
since sodium is highly electropositive hence there is high bond energy between sodium and
carbonate thus is becomes stable.
(c) (i) MgC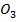 will release C
will release C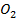 upon heating which will turn lime water milky.
upon heating which will turn lime water milky.
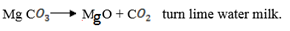
(ii) AlC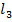 is a Lewis acid, hence it can react with a base to give a salt and HCl. But NaCl cannot react with a base
is a Lewis acid, hence it can react with a base to give a salt and HCl. But NaCl cannot react with a base
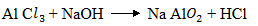
(iii) Cu (N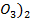 will form a brown ring due to formation of N
will form a brown ring due to formation of N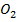 but CuS
but CuS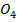 does not.
does not.
5. (a) (i) Fe C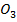 and Al C
and Al C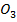 have never been isolated due to the small size of Fe and Al hence they have high polarization power. Hence they will decompose as soon as they are formed.
have never been isolated due to the small size of Fe and Al hence they have high polarization power. Hence they will decompose as soon as they are formed.
(ii) Lime water is used to test the presence of C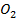 since calcium carbonate is insoluble hence the it becomes milky.
since calcium carbonate is insoluble hence the it becomes milky.
(iii) The reaction between sodium bicarbonate and copper (ii) sulphate, effervescence is observed due to formation of carbon dioxide
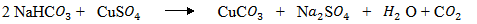
(iv) Lead hydroxide ppt dissolves in sodium hydroxide due to the formation of a soluble complex compound
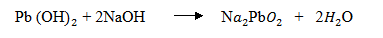
(b) (i) 1st method is by reacting copper carbonate with HCl
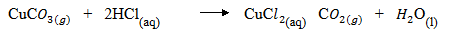
2nd method is by reacting Cu (OH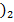 with HCl
with HCl
(iii) ZnO is amphoteric hence it reacts with both acids and bases
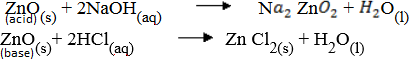
(c) Uses of CaO
 Manufacture of cement
Manufacture of cement
 Used as a drying agent for ammonium
Used as a drying agent for ammonium
 Lining of furnaces
Lining of furnaces
 Formation of slag in Blast furnace
Formation of slag in Blast furnace
 Making Ca
Making Ca which interns is used in making methane
which interns is used in making methane
6. (a) (i) Heat of reaction is the enthalpy change when number of moles of reactants as represented by a balanced chemical equation change into products.
Bond energy is the amount of energy required to break 1mole of a bond
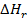 = –
= – 
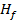 +
+ 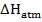 + 4
+ 4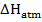
4 (C – Cl) = (-135.5) + 715 + 4(121.1)
C – C l = 333.725 KJ mol-1
(c) (i) Solution:
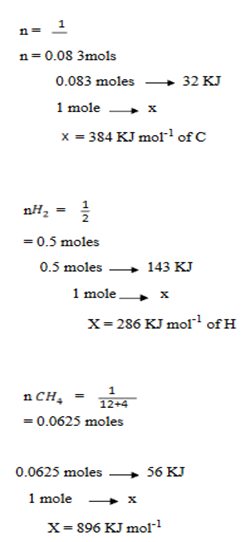

(ii) Solution:
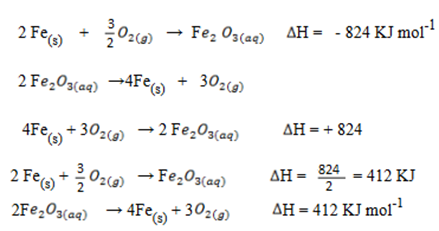
7. Define the following terms
(a) (i) Hydrogen spectrum
Series of arrangement of various wavelength of radiation formed when hydrogen atoms are exposed under high voltage electric discharge in
a discharge tube.
(ii) Convergence Limit
Is the point at which the distance from the nucleus is so large such that electron moving beyond this point cannot go back to its ground state:
(iii) Degenerate orbitals:
. These are orbitals with equivalent energies
. They share the same azimuthal quantum number.
(b) (i) Solution:
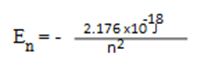
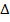 E =
E = 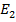 –
– 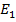
= – 2.176 x 10-18 (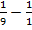 )
)
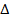 E = 1.934 X 10 -18 J
E = 1.934 X 10 -18 J
(ii) For shortest wavelength, 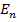 , = n =
, = n = 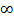
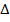 E =
E = 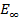 –
– 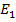
= -2.176 x 10-18 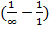
 E = 2.176 x 10-18
E = 2.176 x 10-18
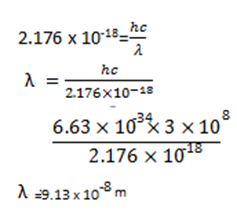
(c) Solution
n = 2 l = 1 ml = -1 ms = – 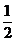

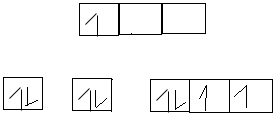
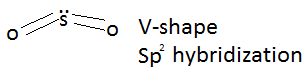
VSEPR – Valency shell electron pair repulsion. We use lines to indicate the bonds
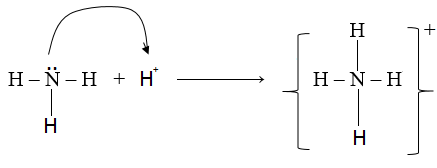
8. (a) Dative bond
It a type covalent bond in which one atom contributes both electrons
Van – Der Waals force is the dipole-dipole interaction between two non polar atoms.
London forces are temporary dipole dipole interactions.
Experiment:
A: Is a solution made by dissolving 4.28g/ 500 cm3 of MI of distilled water
of distilled water
B: Is 10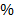 KI solution
KI solution
C: Is dilute 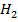 S
S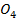
D: Is 0.12M solution Na2 S2 O3 . SH2 O
S: Is starch indicator solution
THEORY:
In acidic media Iodate ions (I 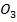 ) reacts with excess Iodine ions (
) reacts with excess Iodine ions (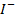 ) to liberate Iodine according to the following reaction
) to liberate Iodine according to the following reaction
I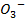 (aq) + 6
(aq) + 6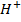 (aq) + 5
(aq) + 5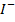 → 3
→ 3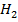 O + 3
O + 3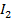 ——————- (1)
——————- (1)
The iodine l liberate with a standard solution of thiosulphate ions according to the following reaction equation.
2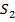
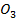 2- +
2- + 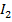
 →
→ 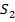
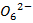 (aq) + 2I–(aq) ——————— (2)
(aq) + 2I–(aq) ——————— (2)
Procedure:
1. Pipette 25 cm3 of solution A into titration flask
2. Using measuring cylinder add 5 cm3 of solution B into titrating flask containing solution A
3. To the resultant solution in (2) above add 5 cm5 of solution C using measuring cylinder
4. Titrate the resulting solution in (3) above with a standard solution D till the colour becomes pale yellow. Then add few drops of solution S and continue to titrate till the blue colour just
disappears.
5. Repeat the titration 3 times
Results:
The volume of pipette used was 10cm3
The volume of burette used was 50cm3
Burette reading
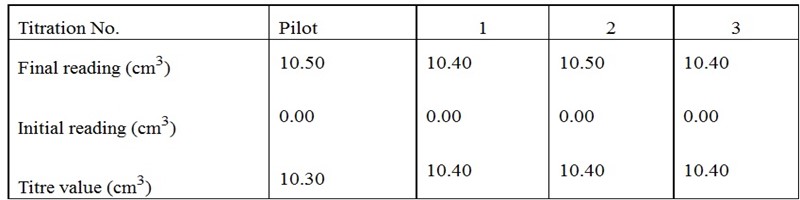
10cm3 of solution A requires 10.40 cm3 of solution D for complete reaction
ANSWER THE FOLLOWING
(a) Write down half reaction equation to show oxidation and reduction taking place in the reaction (1) and (2)
(b) Calculate the number of moles of D used during titration
(c) Calculate the number of moles of A used during titration
(d) Calculate molarity of solution A’
(e) Calculate the R.A.M of metal M in the formula MI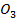
I = 127, 0 = 16
ANSWERS:
(a) Half reactions in (1) and (2)
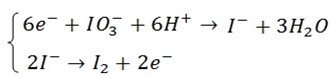
And also
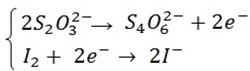
(b) Solution:
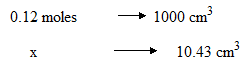
x = 1.25 x 10-3 moles of D was used