Conditions of hydrogen bonding
(i) The highly electronegative atom should be small in size
(ii) The more electronegative atom must have a lone pair
(iii) Hydrogen should be bonded to one of the most electronegative atom N, O, F
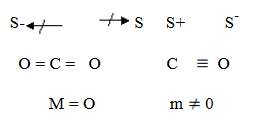
CO2 is non polar since its dipole moment cancel each other since it is symmetrical. But CO is polar since it has a dipole moment caused by electronegativity difference between carbon and oxygen.
Na and K
- Na has higher Boiling Point since it has stronger metallic bond than K
- Ca has a small size with high charge (+2) hence high polarizing power to C
edu.uptymez.com
Thus CaC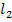 is more covalent. N
is more covalent. N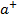 has high size with low charge (+1) this results to low p.p to CC– hence NaCl is ionic.
has high size with low charge (+1) this results to low p.p to CC– hence NaCl is ionic.
1. 9. (a) Give the meaning of second order reaction and derive the units of its rate constant
(b) The reaction between Br 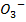 and B
and B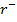 in acidic medium is given by the equation.
in acidic medium is given by the equation.
Br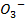 (aq)– + 5B
(aq)– + 5B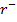 (aq) + 6
(aq) + 6 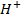 (aq) → 3B
(aq) → 3B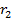 (l) +
(l) + 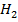 O(l) .
O(l) .
The following table gives the results of four different experiments
| Experiment. No | 1 | 2 | 3 | 4 |
Br 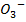 – –
Br– H+ |
0.2
0.2 0.2 |
0.4
0.2 0.2 |
0.4
0.4 0.2 |
0.2
0.2 0.4 |
| Initial rates | 1.56×10-2 | 2.56×10-2 | 5.12×10-2 | 5.12×10-2 |
edu.uptymez.com
Calculate:
(i) Order of reaction with respect to each reactant
(ii) The value of rate constant
2. 10. (a) Briefly explain the meaning of:
(i) Metallurgy
(ii) An ore
(b) Describe the essential steps used during extraction of tin(Sn) show clearly the reaction equations
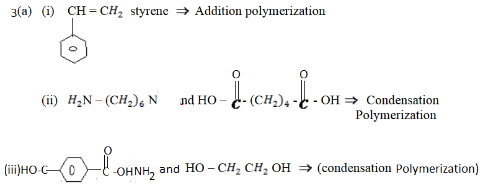
3. (c) Indicate the structure of monomers and polymerization method which is likely to be used in making of each of the following commercial polymer
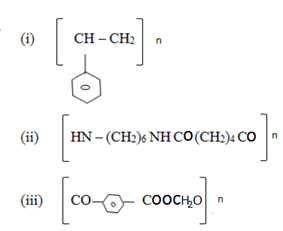
(d) Describe the preparation of benzanamine benzene
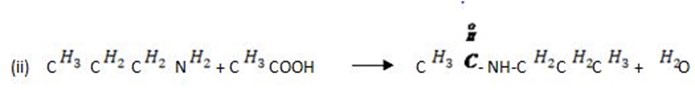
(e) Write the chemical equation to show how propanamine reacts with
(i) Mixture of trichloromethane and potassium hydroxide
(ii) Ethanoic acid
(iii) Chloromethane
ANSWERS:
9.(a) Second order reaction is the reaction in which the rate of reaction is proportional to the Second power of concentration of a single reactant
or first powers of concentration of two reactants.
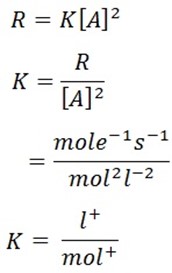
(b) Solution:
(i) R = K [Br O3–] x [Br–] y [H] 2
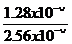
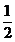 =
= 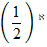
K= 1
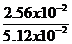 =
=
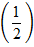 =
= 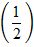 y
y
y = 1
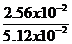 =
= 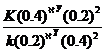
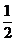 =
= 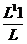 X
X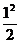
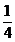 =
= 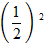
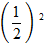 =
= 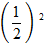
Z = 1, Y = 1, Z = 2
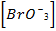 I,
I, 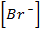 1
1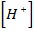 2
2
(ii) Solution:
R = K 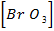
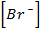
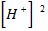
1.28×10 –L = K 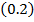
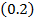
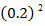
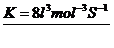
10. (a) Ore
(b) Solution:
The ore in which tin is extracted is called cassiterite (Sn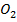 )
)
Concentration of ore:
Cassiterite is pulverized in ball mills. Magnetic impurities such as Fe and Mn are separated out by magnetic separation while other
impurities are removed by Wilfley’s table or hydraulic classifier method. This is done in a reverberatory furnace excess supply of air where
volatile impurities are given out. Impurities of sulphur and arsenic are volatized away.
M + 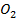 → M
→ M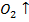
M = As, 5b
S + 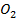 → S
→ S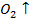
Smelting:
In this process, there is reduction of tin oxide to tin using coal. Temperature should be around 1200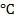 to 1300
to 1300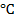
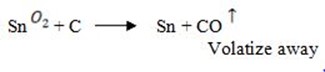
But some of the tin oxide will give SnO which will react with silica to form slag

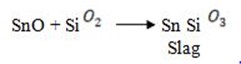
Purification:
This is done in a chamber with no entrance of oxygen
(b) Solution:
N N
N
 + H N
+ H N
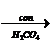
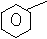

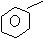
Benzene benzanamine
(c) Solution
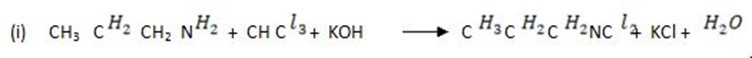
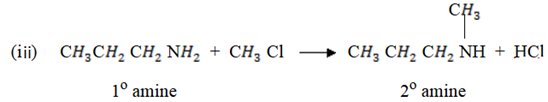
1. 11. (a) (i) State the partition law
(ii) Write down the conditions for the law above to hold
(iii) The partition coefficient for iodine between water and C at 20
at 20 is 2.43×10-3 .A 100 cm3 sample of solution of iodine in
is 2.43×10-3 .A 100 cm3 sample of solution of iodine in 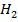 O of conc 1.00×10-3 M is shaken with 10.00 cm3 of C
O of conc 1.00×10-3 M is shaken with 10.00 cm3 of C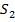 . What fraction of iodine is extracted by C
. What fraction of iodine is extracted by C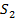 .
.
(b) Define precisely the following terms:
(i) Standard molar enthalpy of formation
(ii) Heat of solution of a substance
(iii) Ionization energy
(c) (i) State Hess’s law of constant heat summation
(ii) Calculate the standard heat of formation of carbon-monoxide if standard heat of combustion of carbon and carbon monoxide are 393 KJ
mol-1 and – 285 KJ mol-1 respectively.
(iii) Determine the enthalpy change for the isomerization reaction:
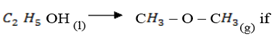
The heat of formation of 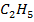 OH is = 276 KJ mol-1
OH is = 276 KJ mol-1
The heat of combustion of C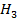 – O – C
– O – C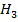 is = 1456 kJmol-1
is = 1456 kJmol-1
The heat of formation of 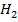 O is =284 KJ mol-1
O is =284 KJ mol-1
The heat of combustion of carbon is =394 KJ mol-1
2. 12. (a) Explain the meaning of the following and give one example:
(i) Nucleophilic addition reaction
(ii) Nucleophilic substitution reaction
(iii) Elimination reaction
(b) How can you distinguish the following compounds
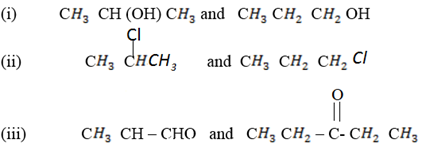
(c) Illustrate the preparation of the following compounds
(i) Propanoic acid from ethane
(ii) Propan – 2 – ol from propan -1-ol
(iii) Propyne from ethyne
13.(a) Explain the following with vivid example and relevant chemical equations.
(i) Li and Mg are more related chemically than Li and Na although both Li and Na are group I
(ii) F is the most oxidizing element of all in the periodic table
(iii) Electron affinity of F is unusually low
(b) Distinguish between the following:
(i) Coordination number and oxidation number
(ii) Paramagnetism and ferromagnetism
(iii) Strong ligand and weak ligand
ANSWERS: