3. LAND POLLUTION
This is contamination of land surface through damping , urban wastes , industrial wastes , mineral exploitation and misusing the soil by harmful ,agriculture process.
Causes 0f land pollution
- Increase in urbanization is major cause of land pollution.
- Construction uses up forest. This leads to the exploitation and destruction of forests.
- Disposal of non – biodegradable wastes included containers, bottles and cans made of plastics, used cars and electronics goods used to the pollution of land.
edu.uptymez.com
EFFECTS OF LANDS POLLUTION
- Makes places look dirty due of tonnes and tonnes domestic wastes dumped without proper disposal of them.
- Land pollution affects respiratory system of human being.
- Land pollution has serious effects on wildlife. Flora which provides food and shelter to wild life destroyed.
edu.uptymez.com
Prevention of Land pollution
- People should be educated and made aware about the harmful effects of littering.
- Items used for the domestic purpose should be reused or recycled.
- Inorganic matter such as paper , glass , plastics and metals should be reclaimed and then recycled.
edu.uptymez.com
Questions:
1. Industries are among the leading sources of air pollutant.
(a) Name four substances from industries which contribute to air pollution.
(b) Explain two other sources of air pollutants.
(c) Give 3 effects of air pollution.
(d) Explain how industrial worker can be protected against harmful chemical fumes.
2. (a) How is ozone formed?
(b) What are the causes of depletion of ozone layer?
(c) Explain harmful effects of depletion of ozone layer.
3. What is green house effect and what are its effects.
4. Write short notes on:-
(i) Acid rain.
(ii) Smog.
(iii)Environmental effect caused by mining.
5. What do you understand by the term Eutrophication and its causes? – How does it threaten the development of marine life?
ANSWER:
Q1. (a) Substances;-
. Carbon dioxide.
. Carbon monoxide.
. Methane.
. Sulphur dioxide.
(b) Other sources:-
. Burning fuels from car.
(c) Depletion of ozone layer:-
. Spread of air borne diseases E.g. Tuberculosis.
. Acid rain.
. Global warming.
(d) Industrial worker can be protected against harmful chemical fumes by:-
. using protective masks to protect them from harmful fumes.
. Good ventilation systems in industries i.e. air circulation.
. Close chambers for chemical processes.
2. (a) Ozone layer formation;-
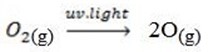
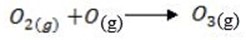
(b) Causes of depletion of ozone layer
(a) Natural destruction.
(b) Artificial destruction.
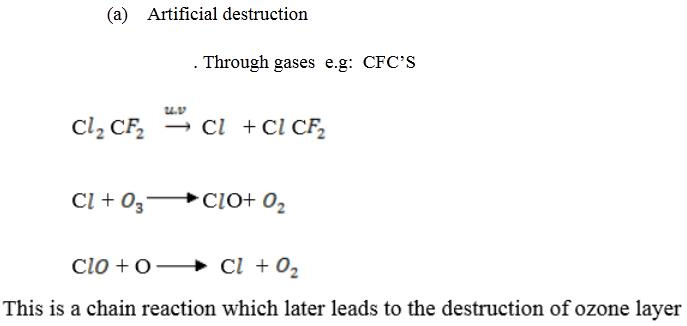
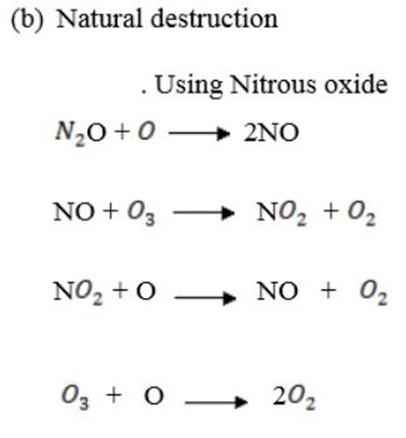
(c) Harmful effects of ozone layer depletion
. It may result to skin cancer due to UV-rays.
. Damage immune system leading to increase in viral infection.
. Damage or death of marine plants and animals which are important to human survival.
3. – Green house effect
Is the process of trapping of heat in the atmosphere. This result in the overall increase in the average temperature of the Earth. The gases are the one’s which
trap the heat in the atmosphere.
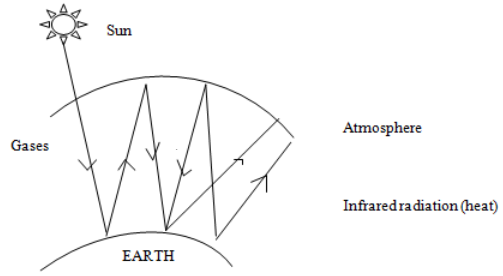
– Effects of green house:–
. Leads to global warming. Ice caps will melt and this leads to increase in sea level which in turn lead to flooding (islands may disappear)
. Change in weather patterns (unpredictable weather)
4. (i) Effects of Acid Rain;-
On Humans
. Irritation of the respiratory system.
. People with bronchitis become highly affected.
On the Soil
. Soil not having proper liming becomes highly acidic.
. Acid rain may cause corrosion on buildings.
. It can cause the death of micro organism in water bodies or on the land.
Reactions for the formation of acidic rain
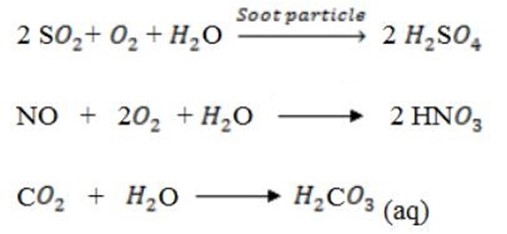
4. (ii) Smog: Contains soot, sulphur dioxide and other gases. It is usually found in cities.
5. Eutrophication:-
Is the process of adding of healthy adequate nutrition to water bodies or Addition of artificial or natural substances such as nitrates or phosphate through water
runoffs sewages etc. This increases number of marine plants such as phytoplanktons.
Causes of Eutrophication
- It is due to adding of nutrients such as Nitrates phosphates.
- Runoffs from sewage.
- Overflow of sanitary sewers.
- Runoffs from industries.
- Untreated sewage.
- Overusing of pesticides and fertilizers.
- Cultivation near water bodies.
edu.uptymez.com
Effects of Eutrophication
. Decrease of biodiversity
Death of marine organisms. This is because of depletion of oxygen. When the phytoplanktons die, they decompose and marine organisms use up oxygen to
decompose them. Hence the concentration of oxygen in water decreases which causes death of the marine organisms.
. Toxicity effect.
. Decrease in water transparency.
Questions:
1. Explain the meaning and significance of soil colloids.
2. Discuss the properties of soil colloids:
. Surface area.
. Electric charge.
. Ion exchange.
3. Explain the mechanism of ion exchange soil.
4. Describe cation exchange capacity of soil.
5. Calculation of percentage base saturation of a soil sample.
1. 6. (a) Why are the configurations of copper and chromium peculiar?
(b) Would you classify scandium and zinc as transition metal? Give the reasons for your answer.
(c) When is copper considered as transition metal?
(d) When does it not show transition behavior?
7. (i) Why are the elements with atomic numbers 21 to 30 classified together as a series in the periodic table?
(ii) List the main characteristics of these elements and their compounds
8. Explain the following observations:-
(i) Water molecules readily coordinate with cations of the above series but Hydronium ion (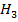 O+ ).
O+ ).
(ii) Addition of excess silver nitrate to an aqueous solution containing 0.01mole of Co C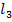 . 6 N
. 6 N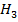 leads to immediate precipitation of 0.03moles of silver chloride. However, similar addition to an aqueous solution containing 0.01mole of Co C
leads to immediate precipitation of 0.03moles of silver chloride. However, similar addition to an aqueous solution containing 0.01mole of Co C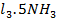 leads to immediate precipitation of only 0.02 moles of silver chloride.
leads to immediate precipitation of only 0.02 moles of silver chloride.