WATER
Water is a very important compound which is essential for the substance of all living things.
Occurrence of Water
The water on the earth occurs in three main states;
1. Solid
example: Ice, snow, hail.
2. Liquid
example: dew, rain.
3.Vapor
example: mist, steam, clouds.
- About 97% of all the water on the Earth is salty water while only 13% is fresh water.
edu.uptymez.com
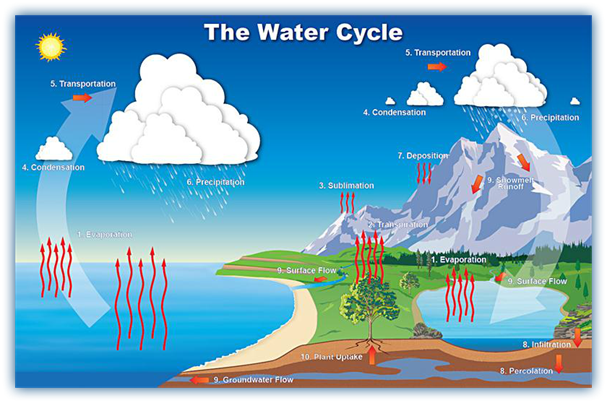
A cycle is a number of change which come back to the starting point. Water is never lost but it is continuously recycled around the globe in a system called water cycle.
The water cycle is made up of 4 main stages:
- Evaporation
- Condensation
- Precipitation
- Collection
edu.uptymez.com
Physical properties of water
- It is colorless, odorless and tasteless.
- It is only substance that occurs naturally in all the three states of matter.
- Pure water freezes at 0cºand boils at 100ºc.
- It is universal solvent because it can dissolve more substance than any other liquid.
- It has a high specific heat index because it can absorb a lot of heat before it begins to get hot.
- It is miscible with many liquid for example ethanol.
edu.uptymez.com
Chemical properties of Water
- Pure water is neutral , it is neither acidic nor basic.
- Cold water reacts with some metals such as potassium, sodium and calcium to form metallic hydroxide and hydroxide and hydrogen gas.
edu.uptymez.com
Example:
Word equation
Cold water + Potassium → Potassium hydroxide + Hydrogen
2H2O(l) + 2K(s) → 2KOH(s) + H2(g)
Calcium + Cold water → Calcium hydroxide + Hydrogen
Ca(s) + 2H2O(l) → Ca(OH)2(s) + H2(g)
3. Steam (water vapor) reacts with some metals such as zinc , Timonium and iron to produce metallic oxide and hydrogen gas.
Iron + Water vapor → Iron(iii)oxide + Hydrogen
2Fe(s) + 3H2O(g)
→ Fe2O3(s)
+ H2(g)
Zinc + Water vapor → Zinc oxide + hydrogen
Zn(s) + H2O(g) → ZnO(s) + H2(g)
WATER TREATMENT AND WATER PURIFICATION
Water treatment: Is the process of making water usable for industrial, medical and other purposes.
The aim is to remove existing contaminants in the water
Treatment process may be physical such as settling, chemical eg.disinfection or biological
Water purification
Is the removal of contaminants from treated water to produce drinking water, pure enough for human consumption. Substances that are removed include bacteria, algae, fungi, minerals and human made chemical pollutants.
Domestic water purification
The common method used at homes in purifying water
- Boiling
- Commercial filters and
- Use of purifiers
edu.uptymez.com
1. Boiling: During the method the water is heated and left for sometimes before heating is stopped. This method helps to kill disease – causing organism. The boiled water is then allowed to cool before being used.
2.Commercial filter: Use 80%, This filters work by having the water pass through a charcoal element that purifies water the filtered water is much clear than the original muddy water.
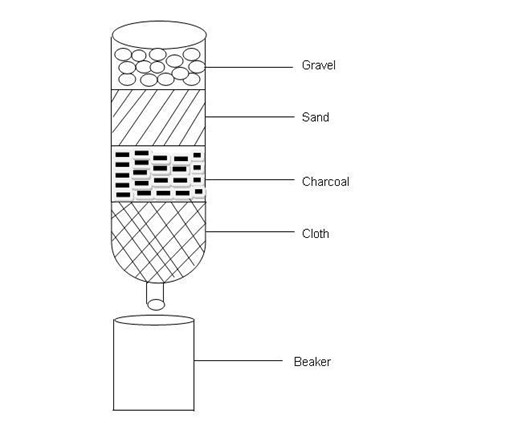
Role of:
Gravel: To trap any floating substances.
Sand: To trap large particles.
Charcoal: To kill some of harmful bacteria.
Clean cloth: To filter the very tiny particles.
3.Uses of purifiers: Chemical purifiers are usually in liquid form. Are commended amount of purifiers is put in a specific of water in a container. The water is shake (stirred) wool then left to set line for at least (20minutes) before it can be sate for drinking. Example of Purifiers area Aqua guard, water guard.
TEST FOR WATER
The presence or absence of water can be established by two methods (regrets);
- Copper (ii) sulphate solution
- Cobalt chloride paper
edu.uptymez.com
1.Copper (ii) sulphate
White anhydrous copper (ii) sulphate turns blue on addition of water.The reason is the formation of a new substance anhydrous copper (ii) sulphate.
2.Cobalt chloride
Blue cobalt chloride paper changes into pink when react with water.
NB: Cobalt chloride test is most common substances than liquid (solution).
URBAN WATER TREATMENT
The water various services before reaching their destiny is substance to see major stages namely;
- Screening
- Reservoir
- Primary filtration
- Secondary filtration
- Disinfection/ chlorination
- Storage
edu.uptymez.com
1. Screening:
Is the stage once water is drawn from its sources, the floating substances are removed.
2. Reservoir
The stage in which water is stored high up, so as it flows through gravitation.
3. Primary filtration
Is a process in which large particles are removed, when they are filtered through courses of sand.
Aluminum sulphate is added to remove smaller particle how?
This is because Aluminum sulphate causes the impurities to chump together and sink to the bottom of contain process is called Coagulation.
4. Secondary filtration
Is a process in which water is passing through finer sand and thus causes removal of smallest particles.
5. Disinfection / chlorination:
Is a process in which chlorine added in a moderate amount to kill harmful bacteria.
6. Storage
This is the final stage where by water is pure and safe enough to be stored for use
Exercise
- The diagram below represent a simple water filter.
edu.uptymez.com
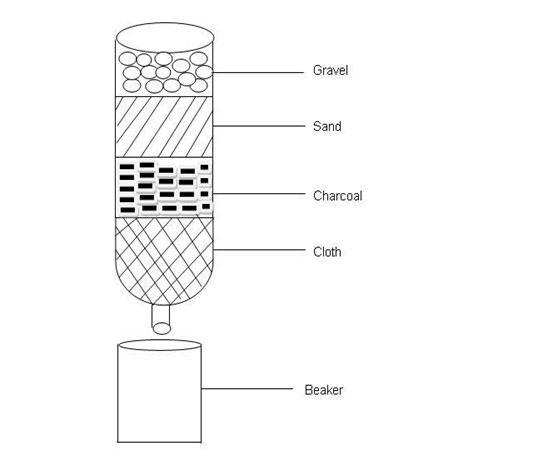
(a) Name the parts labelled A to D.
A- Cloth
B-Charcoal
C- Sand
D- Gravel
(b) What is the importance of each part?
Gravel- To trap any floating substance.
Sand- Is are removed large particles.
Charcoal- Is to kill some of harmful bacteria.
Cloth- To filter the very tiny particles.
(c). What would be the disadvantages of using such as filter to obtain drinking water?
– The disadvantage is that It can cause disease.
IMPORTANCE OF WATER TREATMENT
Reason why water has to be treated:
- Water that has not been treated may contain harmful and other parasites that causes diarrhea , typhoid, cholera other illness.
- Treated water is the best for using in laboratories to ensure accurate result from experiments.
- Treated water is suitable for using in factories to ensure the actual products are Safe for consumption.
- Treated water is more efficient to use for cleaning in industries and domestic setting.
edu.uptymez.com
Conclusion: Untreated water lead to usage of amount of certain substance such as soap for cleaning.
