BASE
A base is a substance which contains the oxide ion or hydroxide ion and reacts with acid to give salt and water only
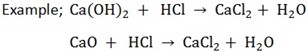
Or
A base is a soluble substance which accepts a proton
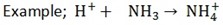
A soluble base is called Alkali
The Alkali’s are
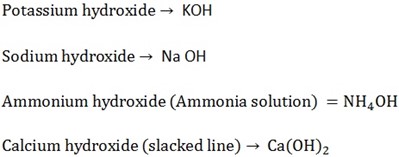
The solution of Ca(OH)2 in water is called lime water
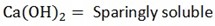
Magnesium hydroxide (milk of magnesia ………Mg(OH)2
It’s also sparingly soluble
The soluble bases when in solution produce OH- as their only negative ion
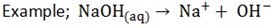
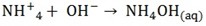
PROPERTIES OF BASES
1. Alkalis have a soapy feeling (slippery)
2. Alkalis have bitter taste
3. Alkalis turn red to blue litmus paper
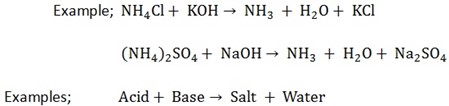
4. They react with acids and water only
Question: which bases combine to form NaCl?
5. Alkalis yield ammonia when warmed with ammonium salts
USES OF BASES

QUESTION:
Calculate percentage of hydrogen in compound (HCL)
H + Cl
1 + 35.5 = 36.5 (atomic masses)
H% = 1/36.5 x 100%
Cl% = 35.5/36.5 x 100%
What kind of equation is?

Answer: Special case reaction
ACIDS
These are the substances which when in solution produce hydrogen ions as their only positive ions
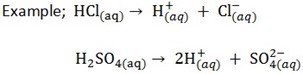
An acid is a substance which turns blue litmus to red and which contains hydrogen replaceable by a metal
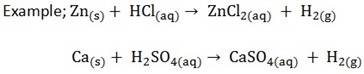
TYPES OF ACIDS
There are two types of acids i) Mineral acids
ii) Organic acids
A: Mineral Acids
These are derived from substances formed from minerals, for example; from minerals
|
Acid |
Formula |
Source |
Formula |
|
Hydrochloric acid |
HCl |
Common salt |
NaCl |
|
Sulphuric acid |
H2SO4 |
Sulphur |
S |
|
Nitric acid |
HNO3 |
Chilean salt petre |
NaNO3 |
|
Carbonic acid |
H2CO3 |
Limestone |
CaCO3 |
edu.uptymez.com
The mineral salts that can be derived from the oxides are called oxy – acids (from the acidic/ non – metal oxides)
B. ORGANIC ACIDS
These are derived from plants and animals source animal source (organic sources)
Example;
|
Acid |
Source |
|
Lactic acid |
Sour milk |
|
Acetic acid |
Vinegar |
|
Tartaric acids |
Grapes |
|
Citric acid |
Citreous fruits |
edu.uptymez.com
STRENGTH OF ACIDS
Strength of acids are classified into two i) Strong acids
ii) Weak acids
STRONG ACIDS
These are acids which ionize completely in aqueous solution
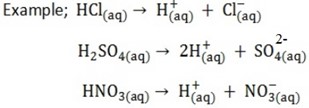
WEAK ACIDS
These are acids which ionize partially in aqueous solution
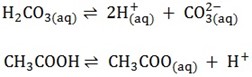
All organic acids are weak
QUESTION;
Is phosphoric acid a strong or weak acid? What are strong bases or weak bases?
PROPERTIES OF ACIDS
1. Have sour taste
2. Action on indicators
Indicator is a substance which changes color in acids or bases
Example; litmus, methyl orange, phenolphthalein etc
|
Indicators |
Color in acids |
Color in alkali |
|
Methyl orange |
Pink |
Yellow |
|
Phenolphthalein |
Colorless |
Pink |
|
Bromothymol blue |
Yellow |
Blue |
|
Litmus |
Red |
blue |
edu.uptymez.com
3. Corrosive action
A concentrated acid is a water molecule aspires. Acid dries any substance of water content in vigorous action, thus burns skin of animals, leaves and stems of plants.
4. Action with metal
Any metal which is above hydrogen in the activity series will replace hydrogen from the acid. The products of these reactions are salt and hydrogen gas
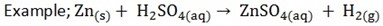
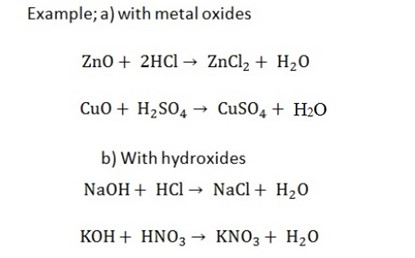
5. Action with metal oxides and hydroxides to form salt and water only
6. Action with carbonates and hydrogen carbonates (bicarbonates)
Acids react with carbonates and bicarbonates producing salt, water and CO2
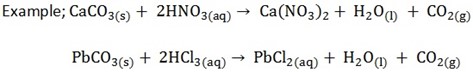
7. Action with sulphates
When acids react with sulphates, SO2, water and salts are produced
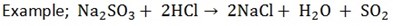
BASICITY OF ACIDS
The basicity of an acid is the numbers of hydrogen ions (H+) produced by one mole of an acid in aqueous solution
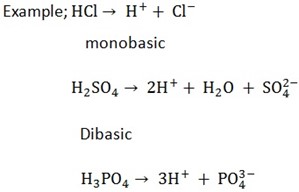
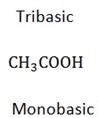
NB: All organic acids are monobasic
PH SCALE;-
It isn’t possible to know exactly how strong an acid or base is by using an indicators. The term PH is used to describe or show the exact strength of an acid or base. It shows the number of hydrogen ion concentration
The PH scale can be represented diagrammatically as show below;
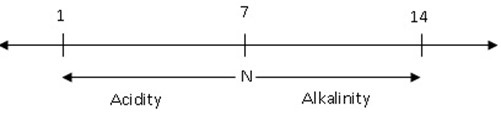
1-4 Strong acid
5-6 Weak acid
7 Neutral
8-9 Weak alkali
10-14 Strong alkali
USES OF ACIDS
Acids have the following uses;
a) Preparation of salts, for example; from fertilizers
From bases / metals.
Ca(OH)2 † 2HCl → CaCl2
† 2H2O
NH4OH + H2SO4 → (NH4)2SO4 + 2H2O
b) Preparation of other acids;
e.g.Pb(NO3)2 + H2SO4 → PbSO4 + 2HNO3
Example;
c) Manufacture of artificial silk
d) Clearing of metals, acids can combine with them to form salts can be easily removed out
e) Necessary for digestion of protein in the stomach.
SALTS
Definition: A salt is a compound consisting of a positive metallic ion (or) and negative ion derived from an acid
Types of salts;
I) Acidic salts
ii) Normal salts
iii) Basic salts
- Acidic salts
edu.uptymez.com
This is the salt which contains part of the hydrogen ions of an acid
Example; Sodium hydrogen – sulphate
Calcium hydrogen carbonate
2. Normal salts
These are the salts which contain neither H+ ions from the acid nor O2- or OH– ions from the base
Example; NaCl, MgCl2,MgSO4 ,FeCl2 ,CaCO3
3. Basic salts
These are salts containing or ions from the base
Example; MgOHCl, ZnOHCl
Magnesium hydroxyl chloride and zinc hydroxyl chloride
O is incorrect because oxygen exists in molecule state but not in atomic
In previous salts was classified according to the nature of salts but in terms of solubility there are two types of salts (i ) Soluble salt ( ii ) Insoluble salt.
SOLUBILITY RULE states;
Soluble salts are;-
a) All salts of (made of) K, Na and NH+4
Example; KNO3,KCl ,K2SO4 ,K3PO4 ,K2CO3,NaHCO3
This is an acidic salt (because it gives out 2 positive ions of Na and H)
-
All nitrates
Example; KNO3
edu.uptymez.com
ii) All hydrogen carbonates
Example; NaHCO3
b) All chlorides except
i) Silver chloride (AgCl)
ii) Mercury (I) Chloride (HgCl) iii) Lead (II) chloride (PbCl2) which is soluble in hot water
N.B: In reactions the compound above are considered as solids (s)
c) All sulphates except
i) – Barium sulphate.
ii) – Lead (II) sulphate.
iii) – Calcium sulphate is slightly soluble in water.
Insoluble salt are;
a) All carbonates except those of K, Na, NH4
b) All hydroxyl except those of K, Na, and Ca (which is slightly soluble in water)
Salt of strong acid and bases do not react with water
Solubility rule (summary)
i) All salts of K, Na and are soluble
ii) All nitrates are soluble
iii) All sulphates are soluble except those Ba, lead (II) and Ca
Vi) All chlorides are soluble except those Ag, lead (ii) and mercury (x)
All carbonates and hydroxides are insoluble except those of K, Na,
NB: – All acids are soluble unless when concentrated (aq)
– All oxides are labeled by solids (s)
Examples;
METHODS OF PREPARING SALTS
A: SOLUBLE SALTS
i) Direct combination of elements
Example; Burning Mg in chlorine gas
Mg + Cl2 → MgCl2
Pass hot Al in Chlorine gas.
2Al(hot) + 3Cl → 2AlCl3
ii) Addition of a metal to a dilute acid. But this method is restricted to metals above hydrogen in the reactivity series.
A metal below hydrogen cannot displace hydrogen
Example;
Zn + 2HCl2 + H2(g)
Na + H2SO4 → Na2SO4 + H2(g)
- Addition of carbonates to a dilute acid.
edu.uptymez.com
Products = salt, water and carbon dioxide.
CaCO3(s) + 2HCl → CaCl2(aq) + H2O(l) + CO2(g)
2. Addition of an oxide to a dilute acid.
CaO + H2SO4 → CaSO4 + H2O(l) + H2O
v) The general method for preparing soluble salts is called crystallization, for example; Preparation of ZnSO4
Procedure;
a) Add excess Zn to dilute in a beaker Add few crystals of and few drops of concentrated to speed up the reaction rate
- If the reaction is still slow warm the mixture
- When the reaction is over, filter and put the filtrate in an evaporating dish
edu.uptymez.com
c) Crystallization
The filtrate is evaporated and cooled. Check if the crystals are formed. If they are formed stop evaporating and leave it for clay.
The water attached or associated to the crystals is called water of crystallization.
ZnSO4.7H2O is called zinc sulphate hepta hydrate.
B. INSOLUBLE SALTS
The insoluble salts are prepared by ionic precipitation also called double decomposition
Here two soluble salts are mixed and react by interchanging their radicals ion forming both soluble and insoluble salts.
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) +2NaCl
Ba2+ (aq) + 2Cl(aq) + 2Na+(aq) + SO42-(aq) → BaSO4(s) + 2Na+(aq) + 2Cl–(aq)
Ba2+(aq) + SO42-(aq) → BaSO4(s)
QUESTION;
Ions to get solid, what is the name of the reaction? It’s a double decomposition reaction or ionic precipitation.
PROPERTIES OF SALTS
1. COLOUR
Some salts are coloured
For example; i) Iron (ii) salts are green
ii) Iron (ii) salts are yellow
iii) Iron (ii) salts are pale blue
iv) Nickel (ii) salts are green
v) Cobalt (ii) salts are pink
The colours of the above mentioned salts are due to the color of hydrated ion
Coloured salts are formed only in the group of transition elements. All elements in first 20 elements are not colored.
Other salts are not colored, for example; salts of Na, K, Ca, Pb, Zn, Al, Mg
2. Hydrolysis
This is the reaction of salts and water giving an acid or alkaline solution
Example:
NH4Cl(aq) + H2O → NH4OH(aq) + HCl(aq)
Na2CO3(aq) + H2O(L) → NaOH(aq) + H2CO3(aq)
a) All salts of weak bases and strong acids hydrolyze to give acid solution
Example; NH4, Cl, FeCl2, CuCl2,Al2(SO4) etc.
b) All salts of strong bases and weak acids are hydrolyzed to give an alkaline solution
Example; Na2CO3 ,CH3COONa, etc.
N.B: Salts of strong bases and strong acids are not undergoing hydrolysis. They only ionize when in solution:
Example; NaCl + H2O → There’s no reaction.
3.
EXPOSURE TO AIR
When salts are exposed to air they either lose water of crystallization or absorb water from the atmosphere
a) Hygroscopic
Is the action of absorbing water from the atmosphere without changing into solution NaCl, anhydrous copper II sulphate (CuSO4) and NaNO3 others are concentrated and copper II sulphate
b)
Deliquescent
This is the action of absorbing water from the atmosphere by a solid to form a solution
Example; MgCl2, CaCl2, FeCl3, Ca(NO3)2
Others are the hydroxides of K and Na
c) Fluorescence
This is the action of giving up water of crystallization of the solid to the atmosphere
Examples;
i) Hydrate sodium carbonate
Na2CO3.10H2O → Na2CO3.H2O + 9H2O
ii) Hydrated sodium sulphate
Na2SO4.10H2O → Na2SO4 + 10H2O
iii) Hydrated magnesium sulphate
MgSO4.7H2O → MgSO4.H2O + 6H2O
4.
HEAT EFFECTS ON SALTS
Different salts behave differently on heating. Most of the hydrated salts loose water of crystallization when heated. The anhydrous salts undergo chemical change when heated
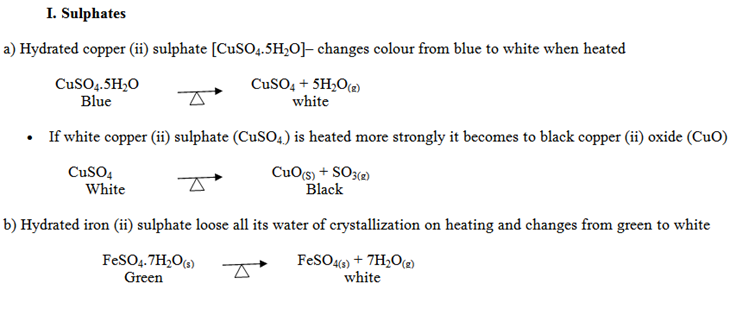
c) When iron (ii) sulphate is heated strongly it decomposes to form black iron (iii) oxide, and SO3 and SO2(g)
2FeSO4 → Fe2O3(s) + SO3(g) + SO2(g)
White black
d) Iron (iii) sulphate when heated strongly it decomposes to form black iron (iii) oxide and SO3 only.
Fe2(SO4)3(s) → Fe2O3(s) + 3SO3(g)
White black
II) Chlorides
All chlorides of metals are hydrated except those of K, Na, Pb, Hg and Ag when heated, chlorides undergo a chemical change called hydrolysis (i.e. they don’t form anhydrous chlorides) in which hydrogen chloride gas (and water involve and a basic salts of chloride or oxide are formed
Example;
MgCl2.6H2O(s)  MgOHCl(s) + HCl(g) + 5H2O(g)
MgOHCl(s) + HCl(g) + 5H2O(g)
AlCl3.6H2O(s)
 AlO3(s) + 8H2O(g) + 6HCl(g)
AlO3(s) + 8H2O(g) + 6HCl(g)
ZnCl2.6H2O(s)
 ZnOHCl(s) + HCl + 5H2O(g)
ZnOHCl(s) + HCl + 5H2O(g)
Ammonium chloride sublimes when heated
NH4Cl(s)
 NH3(g) + HCl(g)
NH3(g) + HCl(g)
III) Carbonates and hydrogen carbonates
Carbonates of sodium and potassium are unaffected by heat (even at very high temperature)
Ammonium carbonate decomposes readily on heating to form NH3(g), CO2(g) and H2O(g)
(NH4)2CO3(s)
 2NH3(g) + CO2(g) + H20(g)
2NH3(g) + CO2(g) + H20(g)
All other carbonates decompose to give oxide and CO2
Al2(CO3)3
 Al2O3 + CO2
Al2O3 + CO2
K2CO3  NO Reaction
NO Reaction
All hydrogen carbonates decompose to give metal carbonates water and CO2 on heating
Mg(HCO3)2 → MgCO3 + H2O + CO2
2NaHCO3 → Na2CO3 +H2O + CO2
IV) Nitrates
Na and K nitrates decompose when heated to give corresponding nitrite and oxygen
NaNO3 → NaNO2 + O2
KNO3 → KNO2 + O2
– Ammonium nitrate decomposes on heating to give dinitrogen oxide and water
NH4NO3 → N2O + H2O
– While the nitrite gives nitrogen and water
NH4NO2 → N2 + H2O
– The metal nitrates (i.e. those of Ca, Mg, Al, Zn, Fe, Pb, Cu) decompose on heating to give the corresponding oxide, nitrogen dioxide and oxygen
Ca(NO3)2(s) → CaO(s) + NO2(g) + O2(g)
Mg(NO3)2(s) → MgO(s) + NO2(g) + O2(g)
– Nitrates of heavy metals (Ag and Hg) decompose to give a metal, NO2 and 02 on heating.
AgNO3 → Ag + NO2 + O2
Hg(NO3)2 → Hg + 2NO2 + O2
V) Hydroxides
The hydroxide of Na and K are stable to heat ie. Don’t decompose on heat even at very high temperature.
All other metal hydroxides decompose on heating to give the corresponding oxides and water vapor
KOH  No Reaction
No Reaction
Mg(OH)2
 MgO + H2O
MgO + H2O
5. SOLUBILITY AND SOLUBILITY CURVES
Solubility of a salt in a liquid is the maximum amount of the salt that will dissolve in 100cm3 of a liquid at any given temperature
Solubility Curve
These are the graphs which show the variation of solubility with temperature
Solubility of a salt increases with the increase in temperature.
The steeper the solubility curve the more soluble the salt and the easier it is to crystallize that salt
SOLUBILITY AND SOLUBILITY CURVES.
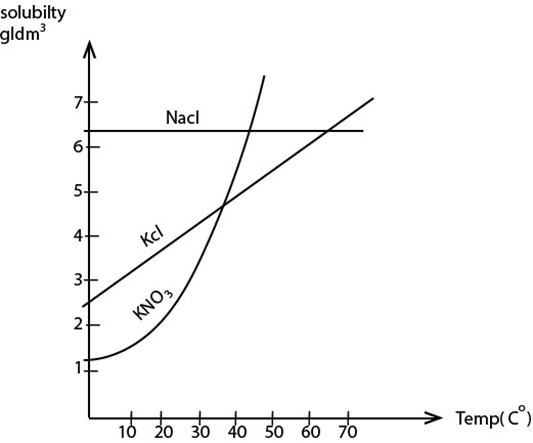
The diagram above is a graph of solubility against temperature. The vertical component is a solubility of substance in gram per dm3 , where by horizontal component is temperature of a substance. The graph drawn in the plane is analysis of two related variables (solubility and temperature). The change of solubility related to change of temperature.
From the accumulated data due to the test of salts NaCl, KCl and KNO3 analyzed in the graph as drawn above.
(i) A graph of NaCl interpret that, it has a constant solubility at any temperature. The increase or decrease of temperature does not affect its solubility.
(ii) A graph of KCl is a smooth linear graph which interpret that for every change of temperature give the effect change of solubility. Therefore KCl is more soluble than NaCl.
(iii) A graph of KNO3 is a curved graph interpret that the change of temperature gives a rapid solubility. Therefore a salt KNO3 is more soluble than NaCl and KCl.
Assignment;
Take a salt of NaNO3(S) to examine its solubility.
Procedure
(i) Measure 1litre of distilled water and pour it into the beaker.
(ii) Measure 1g of NaNO3 by electronic balance and pour into the beaker.
(iii) By heat source, tripod stand, wire gauze, retort stand and thermometer make connection and heat both water and sample salt.
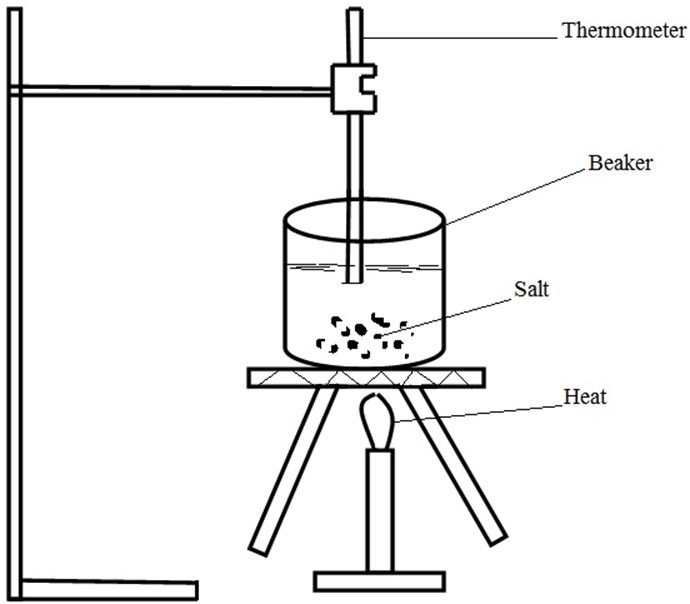
(iv) Watch a disappearance of salt particles until all finish and record the temperature as follows;

(v) Repeat procedure (ii) for except 2,3,4,5,6,7 and 8
(vi) Draw a graph of solubility against temperature.
USES OF SALT IN DAILY LIFE
Salt is essential for life, because it has more than 14000 users in daily. But the common uses categorized into Food, Agriculture,Water conditioning, High way Deicing and Industrial chemicals.
1. FOOD
i) A salt of sodium chloride is mixed with food as flavoring, (common salt).
ii) A salt of sodium chloride (NaCl)is mixed in the food industrial product as both flavoring and preservative.
iii) A salt of sodium bicarbonate (NaHCO3) ‘bicarb’ is used in cooking as raising agent for cakes, bread e.t.c.
Baking powder
Is a mixture of sodium hydrogen carbonate and tartaric acid, The mixture helps to keep the PH neutral.
2. AGRICULTURE
A salt is very important in agriculture since used as land additive nutrients. A fertilizers of Ammonium sulphate (NH4SO4(S)) and Sodium nitrate (NaNO3) salt are used to make land fertility to help healthier growing of crops.
3. WATER CONDITIONING
i) In the purification of urban water (Permutit process),the salt of Aluminum silicate is used to remove permanent hardness of water (Al(SiO3)3).
ii) Sodium carbonate (Na2CO3(S)) called washing soda, is used to make water soft by replacing calcium ions by sodium ions.
iii) Sodium chloride is used in a water softener to generate the ion exchange column.
4. HIGH WAY DEICING
A salt of sodium chloride (NaCl) is mixed with grit and spread on roads to prevent road freezing in cold condition.
5. INDUSTRIAL USES
i) The salt of potassium Iodide (KI) is added to sodium chloride (common salt) to prevent a lack of Iodine in the diet.
ii) The salt of sodium carbonate is used in the manufacture of glass.
