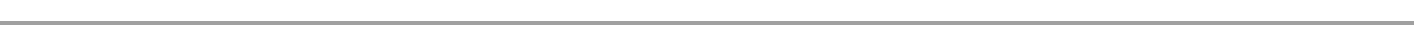NITROGEN AND ITS COMPOUNDS
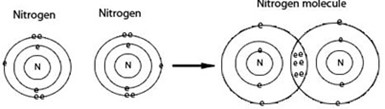
Nitrogen, symbol N, is the chemical element of atomic number seven and electronic configuration (2:5). At room temperature, it is a gas of diatomic molecules and is colorless and odorless. Nitrogen is a common element in the universe, estimated at about seventh in total abundance in our galaxy and the Solar System. On Earth, the element is primarily found as the gas molecule; it forms about 78% of Earth’s atmosphere. The element nitrogen was discovered as a separable component of air, by Scottish physician Daniel Rutherford, in 1772.
Nitrogen gas as the diluents gas
Nitrogen gas is said to be the diluents gas. This is because it reacts with oxygen gas supporting combustion, the reaction between oxygen and nitrogen help to reduce the rate of combustion, rusting. This shows that if nitrogen in the atmosphere was absent, then rusting and combustion could occur faster on the Earth’s surface.
Inert/ unreactive noble property of nitrogen
Nitrogen gas – N2 is unreactive gas and can resemble or be similar to noble gas due to the truth that covalent bond in nitrogen is long and strong to break down hence nitrogen becomes unreactive gas. The bond is triple covalent bond. E.g. N2
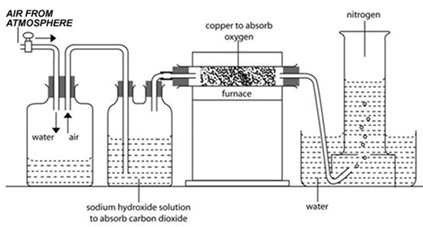
LABORATORY PREPARATION OF NITROGEN
Apparatus – delivery tubes, furnace, beaker, trough, Bunsen burner, 2 wash bottles Chemicals – Air, water, sodium hydroxide Procedure Either: Nitrogen is prepared from the air by removing oxygen and carbon dioxide. Water is used to push air through sodium hydroxide solution (caustic soda solution) which removes carbon dioxide.

The remaining gas is passed over heated copper turnings to remove Oxygen.

Nitrogen is collected over water as it is insoluble in water.
PROPERTIES OF NITROGEN
Physical properties
(a) it is colorless gas without smell
(b) it is a reactive gas
(c) it does not burn / doesn’t support combustion
(d)it is neither acidic nor basic
(e)It is less denser than air
(d)It is insoluble in air
Chemical properties
Nitrogen is inert unlike Oxygen; it reacts under special conditions for example
- It reacts with some metals at very high temperatures forming nitrides e.g. Calcium and magnesium.
edu.uptymez.com
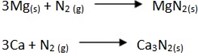
- It reacts with hydrogen to form ammonia (Haber’s process)
edu.uptymez.com

Commercial uses of nitrogen
- Used in the storage of petroleum to avoid the exploding of petrol.
-
Used in the preparation of ammonia gas.

edu.uptymez.com
Common drying agent in laboratory for gas
- Concentration of sulphuric acid
- Anhydrous calcium chloride.
- Calcium oxide (CaO)
edu.uptymez.com
Ammonia gas
Ammonia, or azane, is a compound of nitrogen and hydrogen with the formula NH3. It is a colorless gas with a characteristic of pungentsmell. Ammonia contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to food and fertilizers. Ammonia in both directly or indirectly is also a building-block for the synthesis of many pharmaceuticals and is used in many commercial cleaning products. Although in wide use, ammonia is both caustic and hazardous. The gas form white denser fumes with hydrogen chloride gas.
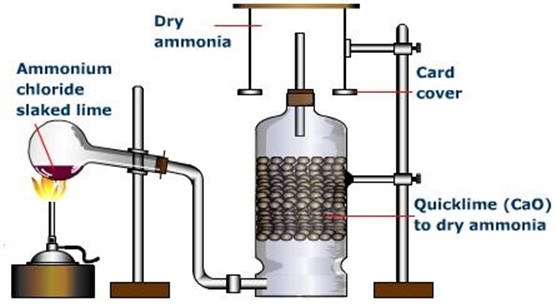
PREPATION OF AMMONIA
Ammonia is prepared by heating a mixture of calcium hydroxide and ammonium chloride. Ca(OH)2(s) + 2NH4Cl(s) → CaCl2(s) + 2H2O(L) + 2NH3(g)
The tube in which ammonia is generated is fixed in a slanting position to prevent the water formed from running back and crack the whole tube. Concentrated sulphuric acid and anhydrous calcium chloride are not used to dry ammonia because they react with it. The gas is passed through fresh quicklime (solid calcium oxide lumps) to effectively dry it. Ammonia is collected by upward delivery as it is lighter than air
Compounds of nitrogen
- Nitric acid ( HNO3)
- Nitrogen monoxide (NO)
- Dinitrogen Monoxide (N2O)
- Nitrogen dioxide.
edu.uptymez.com
Physical properties, chemical properties and uses of ammonia
Physical Properties of Ammonia
- Ammonia is a colorless gas.
- It has a pungent odor with and an alkaline or soapy taste. When inhaled suddenly, it brings tears into the eyes.
- It is lighter than air and is therefore collected by the downward displacement of air.
- It is highly soluble in water: One volume of water dissolves about 1300 volumes of ammonia gas. It is due to its high solubility in water that the gas cannot be collected over water.
- It can be easily liquefied at room temperature by applying a pressure of about 8-10 atmosphere.
- Liquid ammonia boils at 239.6 K (- 33.5°C) under one atmosphere pressure. It has a high latent heat of vaporization (1370 J per gram) and is therefore used in refrigeration plants of ice making machines.
- Liquid ammonia freezes at 195.3 K (-77.8°C) to give a white crystalline solid.
edu.uptymez.com
Chemical properties of ammonia
They include;
1.The combustion of ammonia proceeds with difficulty but yields nitrogen gas and water.
4NH3 + 3O2 + heat → 2N2 + 6H2O
2. Ammonia readily dissolves in water with the liberation of heat.
NH3 + H2O ⇔ NH4+ + OH−
3. Ammonia gets oxidized to nitrogen, when passed over heated metal oxides.
Uses of ammonia
- In the manufacture of rayon and urea
- In the manufacture of fertilizers such as urea diammonium phosphate, ammonium nitrate, ammonium sulphate etc.
- In ice plants, as a refrigerant
- In furniture industry, as a cleansing agent for furniture and glass surfaces.
- In the manufacture of nitric acid by Ostwald’s process.
-
In the manufacture of sodium carbonate by Solvay’s process.
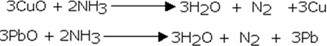
EXPERIMENT TO SHOW THE REDUCING PROPERTIES OF AMMONIA TO COPPER OXIDE- CuO
edu.uptymez.com
Consider the diagram below.
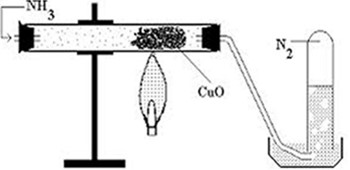
Chemical equation for the reaction

Fountain experiment to demonstrate solubility of the gases e.g. NH3, HCl
Fountain experiment this is an experiment used to show the solubility of the gases.
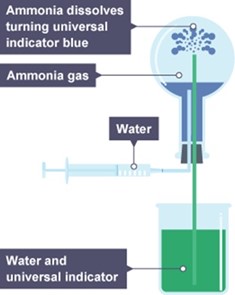
The ammonia fountain is a type of chemical demonstration. The experiment consists of introducing water through an inlet to a container filled with ammonia gas.Ammonia dissolves into the water and the pressure in the container drops. As a result more water is forced into the container from another inlet creating a fountain effect. The demonstration introduces concepts like solubility and the gas laws at entry level.
A different gas of comparable solubility in water, such as hydrogen chloride, can be used instead of ammonia.
If the ammonia is replaced by a liquid vapor, such as water vapor, at a pressure higher than its room-temperature vapor pressure, a similar effect is produced. In this case, the reduction in pressure in the container is due to condensation of the vapor as the container cools to room temperature
Nitric acid
Nitric acid(HNO3), also known as aqua Fortis and spirit of niter, is a highly corrosive strong mineral acid. The pure compound is colorless, but older samples tend to acquire a yellow cast due to decomposition into oxides of nitrogen and water. Most commercially available nitric acid has a concentration of 68%. When the solution contains more than 86% HNO3, it is referred to as fuming nitric acid. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as white fuming nitric acid or red fuming nitric acid, at concentrations above 95%.
Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as pigments in inks and dyes. Nitric acid is also commonly used as a strong oxidizing agent.
Preparation of Nitric Acid
Laboratory preparation
Nitric acid is usually prepared by heating potassium nitrate with concentrated sulphuric acid. This heating is done in a glass retort and vapors of nitric acid are condensed in a receiver, which is cooled by water. The reaction is:

Chemical
1. Potassium nitrate: 30 g
2. Sulfuric acid: 98% 35mL
Procedure
1. Place 30 g potassium nitrate into a round-bottomed flask.
2. Pour 35 ml 98% sulfuric acid and place a stir bar into the flask.
3. Set up a simple distillation apparatus and start heating the round-bottomed flask with a hot plate with stirring. Soak the collecting vessel in a cold-water bath
Consider the diagram below.
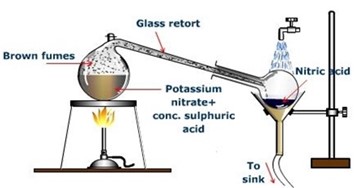

To ensure our yield of anhydrous nitric acid is higher, adding some excess sulfuric acid is very helpful. It makes the final product less water and produces more nitric acid. So weadd equation 2 sulfuric acids into equation 1 potassium nitrate to produce anhydrous nitric acid.
Industrial Preparation
On a commercial scale, nitric acid is manufactured through the Ostwald’s process – the process of catalytic oxidation of ammonia.
Ostwald’s process
The conversion of ammonia into nitric acid in this process is done through the following steps:
Step1
Oxidation of ammonia to nitric oxide
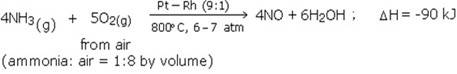
Ammonia is oxidized by air in the presence of Pt catalyst at 800°C to give nitric oxide.
Step 2
Oxidation of NO to NO2
The nitric oxide is oxidized by air at temperature below 100°C, to give nitrogen dioxide (NO2)
Step 3
Formation of nitric acid
Nitrogen dioxide is then converted to nitric acid by absorbing NO2 in water, in the presence of air.
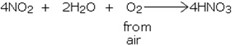
Properties of nitric acid (HNO3)
- It can attack the rubber materials.
- It is unstable so can decompose at room temperature.
- It is yellowish in color, due to the dissolved nitrogen dioxide, NO2 in the acid.
- It is the strong oxidizing agent
- It is a fuming acid.
edu.uptymez.com
Chemical properties of nitric acid
-
Reaction with non metals.

CO2 Gas turns lime water to milky
And extinguishes fire and a reddish brown gas is evolved.
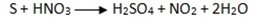
-
Reaction with metal.
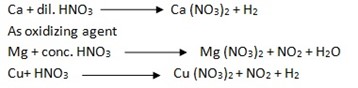
edu.uptymez.com
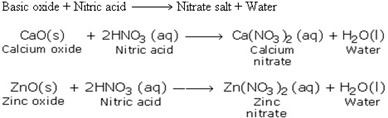
3. Reactions with Basic Oxides
4. Reaction with Bases (Hydroxides)
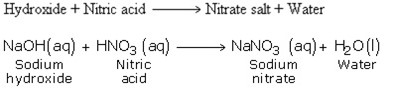
5. Reaction with Carbonates and Hydrogen Carbonates
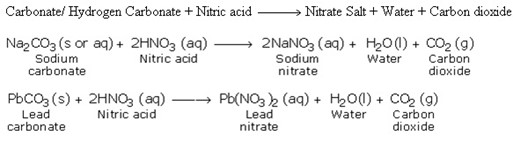
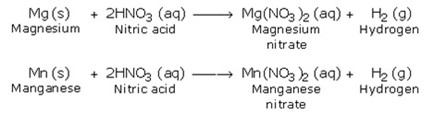
6. Reaction with Metals
Nitric acid usually does not behave as an acid, with metals to form the corresponding salt and liberate hydrogen.
However, magnesium and manganese are the only two metals, which react with cold and very dilute (1%) nitric acid to evolve hydrogen.
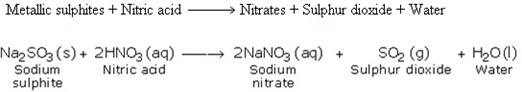
7. Reaction with Metallic Sulphites
Uses of nitric acid
The important uses of nitric acid are as follows:
1) Nitric acid plays a significant role in the manufacture of various products such as:
- Explosives like trinitrotoluene (T.N.T.) nitro glycerin, gun cotton, ammonia etc.
- Fertilizers such as calcium nitrate, ammonium nitrate etc.
- Nitrate salts such as calcium nitrate, silver nitrate, ammonium nitrate.
- Dyes, perfumes, drugs etc. from coal tar products.
-
Sulphuric acid by Lead Chamber process.
2) It is used in the purification of silver, gold, platinum etc.
3) Nitric acid is used in etching designs on copper, brass, bronze ware etc.
4) It is used to prepare “aqua regia” to dissolve the noble elements.
5) It is used as a laboratory reagent.
edu.uptymez.com
NITROGEN MONOXIDE (NO)
Nitric oxide, or nitrogen oxide, also known as nitrogen monoxide, is a molecule with chemical formula NO. It is a free radical and is an important intermediate in the chemical industry. Nitric oxide is a by-product of combustion of substances in the air, as in automobile engines, fossil fuel power plants, and is produced naturally during the electrical discharges of lightning in thunderstorms. This is the colorless gas which turns reddish-brown on the exposure to air. The gas has strong affinity to oxygen, so can react with oxygen from the atmosphere then turn to reddish brown.
Properties of nitrogen monoxide
- It is colorless
- It is neutral to litmus.
- It is odorless.
- It is insoluble in water.
- It is denser than air.
edu.uptymez.com
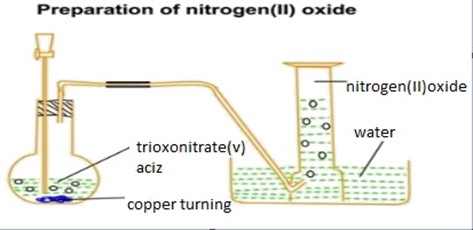
Preparation of nitrogen monoxide in laboratory
The gas can be prepared when nitric acid react with moderate reactive metal.
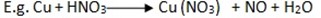
The delivery tube is passed over the water to remove dissolved impurities (nitrogen dioxide)
Consider the diagram below.
Chemical properties of nitrogen monoxide
They include;

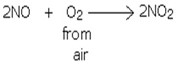
The gas reacts with oxygen gas readily to form nitrogen dioxide gas.
Commercial uses of the nitrogen monoxide
- Used to prepare nitrogen dioxide gas.
- Used to prepare nitric acid in Haber process.
edu.uptymez.com
Nitrogen dioxide
Nitrogen dioxide is the chemical compound with the formula NO2. It is one of several nitrogen oxides. NO2 is an intermediate in the industrial synthesis of nitric acid, millions of tons of which are produced each year. This reddish-brown toxic gas has a characteristic sharp, biting odor and is a prominent air pollutant. Nitrogen dioxide is a paramagnetic, bent molecule with C2 point group symmetry.
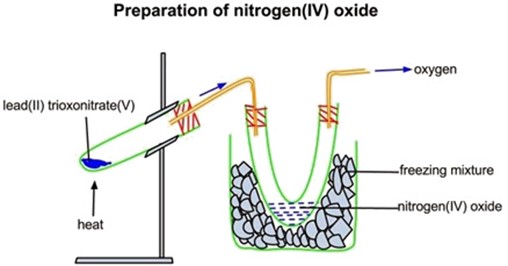
Preparation of nitrogen dioxide gas
The gas can be prepared by heating lead nitrate Pb (NO3)2

Properties of nitrogen dioxide gas
-
Soluble in water and form two acid nitrous acid- HNO2 and nitric acid.

- Have irritating chocking smell.
- Its acidic gas turns dump blue litmus red.
- It is reddish brown yellow gas.
- It is less dense than air.
edu.uptymez.com
Chemical properties of nitrogen dioxide
They include;
-
React with base

-
Action of heat.

On strong heating the gas nitrogen dioxide decompose to produce nitrogen monoxide and oxygen.
Commercial uses of nitrogen dioxide
- Used in preparation of nitric acid
- Used in manufacturing of the salt.
edu.uptymez.com
Differences between nitrogen monoxide and nitrogen dioxide
| Nitrogen dioxide | Nitrogen monoxide |
| It is soluble in water | It is insoluble in water |
| It is reddish brown yellow in color | It is colorless |
| It has irritating chocking smell | It has no pungent smell |
| It is acidic in nature | It is neutral in nature |
| It change dump blue litmus to red | It doesn’t change litmus. |
| Don’t burn air | It burn in air |
edu.uptymez.com