PREPARATION OF THE ALCOHOL.
Ethanol is commercially produced using a process called fermentation. Many other alcohols can be made this way, but are more likely to be produced by synthetic routes – from natural gas, oil or coal.
Fermentation is the process in which yeast breaks down sugar into alcohol and carbon dioxide. Yeast is tiny. The process does not need oxygen, hence it is the form of anaerobic respiration.
These are two types of fermentation
a) Alcoholic fermentation – producing alcohol
b) Lactic acid fermentation – producing lactic acid
The word equation for this process is:
|
Glucose + yeast → alcohol + carbon dioxide |
edu.uptymez.com
Carbon dioxide gas bubbles out of the fermenting solution into the air leaving a mixture of ethanol and water. It’s important that no air is present or the yeast will produce ethanoic acid – the chemical found in vinegar.
Beer and lagers
Barley, hops, water and live yeast produce beers and lagers. The sugar in the mix comes from the spouting barley. Bitter, stout and ale use top-fermenting yeast, while lager uses a variety that sinks to the bottom.
Wine In wine making the sugars come from the flesh of the crushed grapes. The type of wine produced depends on the type of grape used in the process.
Spirits and distillation
Yeast cannot survive in high levels of alcohol, so to create stronger spirits an additional process, distillation, is required. Fermented drinks are distilled to create vodka, rum and other spirits. Distillation relies on ethanol having a lower boiling point than water. When the fermented drink is heated the ethanol vaporizes at 78.5 degrees and the water is left behind (water boils or vaporizes at 100 degrees). The ethanol gas is caught and cooled so it condenses into a stronger concentration of ethanol liquid.
Alcohol can be prepared locally or in the industries
1. Local preparation of the alcohol; raw material – sugar beet, cereals such as (cassava, millet, maize)
b) Industrial preparation of the alcohol; Raw material – maltose sugar – C12H22O11
The enzyme known as the diastase help to covert the starch to maltose; normally the starch containing food are crushed then treated with the steam to extract starch from them. The malt from Barley added in order to convert the starch to maltose, the mixture allowed to stay for about one hour also yeast can be added, and the yeast contains two enzymes maltose and zymase.

Definition: Fractional distillation – is the process used to separate the components of the homogeneous mixtures which differ in the boiling points 9BP) consider the diagram below
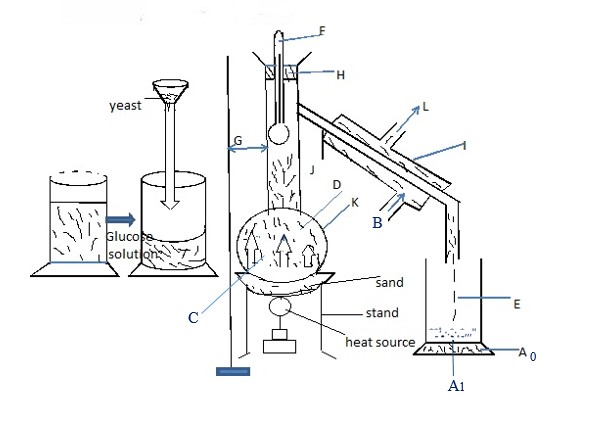
Where
A – Stand for ethanol – C2H5OH K- Round bottomed flask
B – Cold water to distillate ethanol vapors to liquid A1– Ethanol(C2H5OH)
C – Ethanol (780c) and water (1000c) A0-Beaker(Container)
D – Ethanol vapour
E – Ethanol drops
F – Thermometer record temperature
G – Stand and clump
H – Cork
I – Condenser
Note
- The mixture boiled until temperature reach about 80 – 850c then stopped at this point all ethanol become extracted from the mother solution
- The yeast cells are killed by the high temperature
- The yeast contains two enzymes known as Maltose, and Zymase
-
Alcohol can also prepared from the sugar cane
E.g. sugar cane + water yeast → Glucose + Fructose
Sucrose + water → Glucose + Fructose
C12H22O11 + H2O → CH12O6 + C6H2O6
The invertase enzymes connect sucrose to glucose and fructose
PROPERTIES OF THE ALCOHOL
The following are the properties of the Alcohol
1. It is colorless and has odour taste
2. It is soluble in water and can from the homogeneous mixture water
3. It boils at 78oc
4. Purely ethanol is non – electrolyte
REACTION IN ALCOHOL
They includes
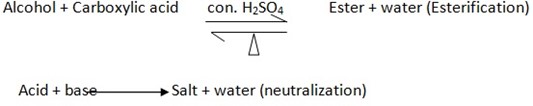
1. Reaction with Carboxylic Acid
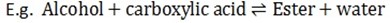
edu.uptymez.com
- Alcohol reacts with carboxylic acid to form “Ester” and water, hence reaction called Esterification reaction
-
Example
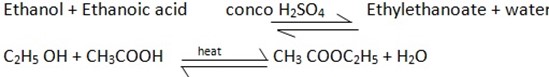
2. Reaction with Electropositive metals
E.g. Alcohol + Electropositive metals salt + hydrogen
Examples
Ethanol + sodium → sodiumethoxide + hydrogen
C2H5OH + Na → C2H5ONa + H2O
Ethanol + Potassium → potassiumethoxide + hydrogen
C2H5OH + K → C2H5OK + H2
3. Oxidizing agent reaction e.g. potassium permanganate – KmnO4 or (O)
E.g. Alcohol + oxidizing agent → Carboxylic acid
Example
Ethanol + (O) → Ethanoic acid
C2H5OH + (O) → CH3COOH
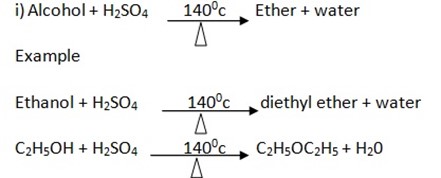
4. Reaction with concentrated sulphuric acid Con. H2SO4 at 1800c/ 1400c
Example
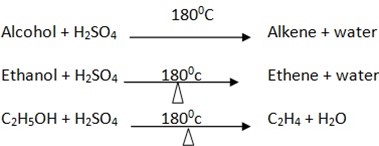
In this reaction acid acts or behave as dehydrating since removed water from the Alcohol
5. Reaction with PCl5, PCl3, SOCl2
E.g. ROH + PCl5 → RCl + POCl3 + HCl
C2H5OH + PCl5 → C2H5Cl + pOCl3 + HCl
ROH + PCl3 → RCl + H3PO4 + HCl
C2H5OH +PCl3 → C2H5Cl + H3PO4 + HCl
ROH + SOCl2 → RCl + SO4 + HCl
C2H5OH + SOCl2 → C2H5Cl + SO2 + HCl
Where
PCl5 – phosphorus pentachloride, PCl3 – phosphorus trichloride
SOCl2 – sulphuric trichloride or vinyl chloride
Note
COMMERCIAL USES OF ETHANOL – C2H5OH
- Manufacture of the spirit (purely spirit) which can be used in the hospital for the injections or saloon after shaving the spirit is used in order to kill the bacteria (sterilization). E.g. Methanol (5%) extracted by the dry distillation of the wood mixed with 95%- ethanol then we obtain methylated spirit which is coloured before being sold to the public this is to avoid the ethanol from being consumed as the drink.
- They can be used for
edu.uptymez.com
- Manufacture of the spirit (purely spirit) which can be used in the hospital for the injections or saloon after shaving the spirit is used in order to kill the bacteria (sterilization). E.g. Methanol (5%) extracted by the dry distillation of the wood mixed with 95%- ethanol then we obtain methylated spirit which is coloured before being sold to the public this is to avoid the ethanol from being consumed as the drink.
- Preservation of the specimen and food
- Fuel and light
- Varnishes, polishes, paint making and removing
- Temporary solvent in the manufacture of the soap, medicine, also to precipitate the chemicals from their aqueous solutions
-
Raw materials for the manufacture of the chemical such as trichloromethane and ethane
3. Manufacture of the Alcoholic drinks egg beer contains 2 – 6% ethanol, made by fermenting mash barley. Wines are made grapes, and yeast for the fermentation grows on the skin of the grapes. Wines contains 10% ethanol
Note: sugar beet molasses – Are the remains of sugarcane
HARMFUL EFFECT OF ALCOHOL
Alcohols have much harmful effects to the body, if taken as a drink. However, the effect of a given amount of ethanol depends on the rate of drinking, the size of the drinker, the alcoholic content of the drink, health and diet of the drinker and other factors.
The noticeable harmful effects of alcohols including:
1) Heavy drinking of alcohol causes shrinkage of the brain cells, reduces power of abstract reasoning and destroys liver cells.
2) Heavy drinking can cause cancer of the liver, stomach and gullet.
3) Heavy drinking can upset digestion and reduce blood cell formation thus causing anaemia.
4) Some of car accidents by drivers is due to heavy alcohol drinking. Don’t drink and drive.
5) Alcohol drinking can be one of the sources of family conflicts, misunderstandings, misuse of money and even divorce.
6) Methanol(methyl alcohol) is quite toxic. Ingestion of as little as 30ml can cause permanent blindness or death.
CARBOXYLIC ACID
edu.uptymez.com
A carboxylic acid is an organic compound that contains a carboxyl group (C(O)OH). The general formula of a carboxylic acid is R-C(O)OH with R referring to the rest of the (possibly quite large) molecule. Carboxylic acids occur widely, and include the amino acids and acetic acid (active ingredient in vinegar).
The general formula
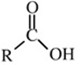
Where
R = CH3 or C2H5 = alky group
Example
R = CH3 = CH3COOH
R = C2H5 = C2H5COOH
Note: Function Group – COOH of carboxylic acid can’t move from the first or last carbon ( c ) atom, this mean functional group is fixed but that of alcohol can move
|
NO OF CARBON |
NAME OF ACID |
MOLECULAR FORMULA |
|
n = 1 n = 2 n = 3 n = 4 n = 5 n = 6 n = 7 n = 8 n = 9 n = 10 |
Methanoic (formic) Ethanoic acid Propanoic acid Botanic acid Pentanoic acid Hexanoic acid Heptanoic acid Octanoic acid Nonanoic acid Decanoic acid |
CH2O C2H4O2 C3H6O2 C4H8O2 C5H10O2 C6H12O2 C7H14O2 C8H16O2 C9H18O2 C10H20O2 |
edu.uptymez.com
CARBOXYLIC ACID
Carboxylic acids are organic acids that are characterized by R-COOH group. The R is an alkyl group. The functional group is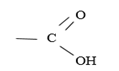
Organic acids have the general formula 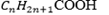
Where n = 1, 2, 3, . . . . 
From the general formula the first five members of homologous series are shown in table below.
Homologous series of carboxylic acid.
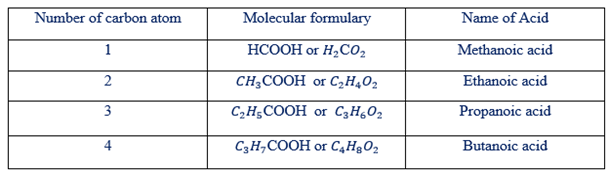
NB It seems that homologous series of carboxylic acid that the carboxylic acid can be represented by using the general molecular formula of 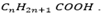 .
.
where n = number of carbon atoms in the compound.
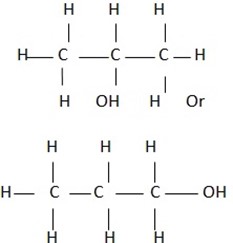
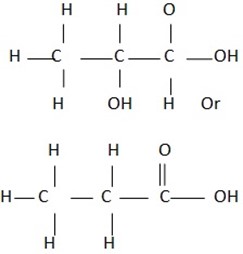
STRUCTURAL FORMULA OF SOME CARBOXYLIC ACIDS
Condensed and open structural formula of carboxylic acid.
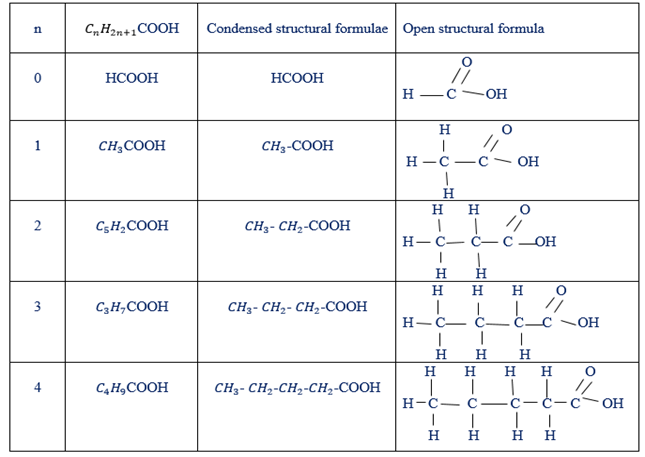
NOMENCLATURE OF CARBOXYLIC ACIDS
In naming carboxylic acids, all rules of naming alkenes are used except the modifications that follows:
(i) The suffix ‘e” of alkanes is replaced by suffix ‘oic” of carboxylic acid.
eg; Alkane Carboxylic acid
- Methane -Methanoic acid
- Ethane -Ethanoic acid
- Propane -Propanoic acid
edu.uptymez.com
(ii) The longest chain of carboxylic acid must includes the carboxylic group
must includes the carboxylic group
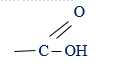
eg: (i) CH3CH2CH2CH2COOH – Pentanoic acid
(ii)CH3CH2COOH – Propanoic acid
(iii) If the substituent groups are attached to the parental chain of the Carboxylic acid, their position must be indicated. Numbering starts the side where by carboxylic group (COOH) is early attached.
eg: 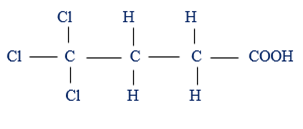 4,4,4 – Trichlonobutanoic acid
4,4,4 – Trichlonobutanoic acid
R-5.7.1.2 Substituted carboxylic acids
Hydroxy, alkoxy, and oxo acids. Some trivial names for hydroxyl and alkoxy acids are retained. The names of carboxylic acids containing an aldehydic group attached to, or a ketonic group contained in the principal chain or parent ring system, are generally derived from the names of the corresponding simple carboxylic acids by adding prefixes such as “oxo-“, “dioxo-“, etc., denoting =O substituents, or “formyl-“, demoting a -CHO substituent.
Example
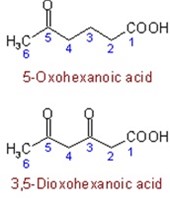
The carboxylic acid also has common which people they know due to daily uses of such carboxylic acid
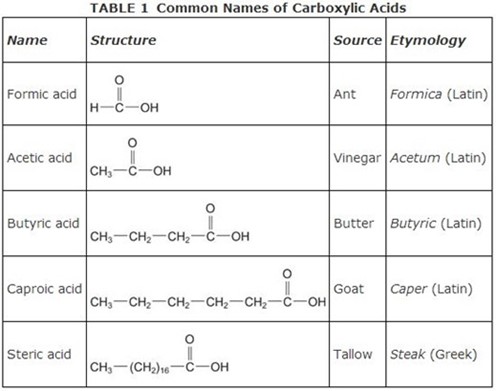
Give the IUPAC names of the following compounds
IUPAC name: 2, 3 – dibromobutanoic acid
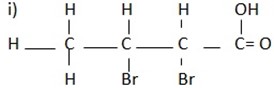
IUPAC name
2 – Chloro, 4, 5 – dimethylhexanoic acid
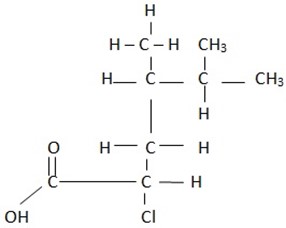
iii) 
Name
2,2 – dimethylpropanoic acid
PROPERTIES OF THE CARBOXYLIC ACID
- They are Acidic in nature so they can react with base then form salt and water
- They have carboxylic group as their functional group – COOH
-
They end up with suffix – “oic”
E.g. Methanoic, Ethanoic
Where
n = 0…..methanoic
n = 1…..ethanoic
n = 3, 4, 5, 6, 7….10
CnH2nO2 where n = 1, 2, 3, 4,
REACTION OF CARBOXYLIC ACID – C00H
edu.uptymez.com
Carboxylic acid are acidic in nature so they can react with base then form salt and water as other acid (mineral acid)
-
CARBOXYLIC ACID AND BASE (Alkali)
E.g. Acid + base → salt + water
Example
edu.uptymez.com
-
Ethanoic + sodium → sodium + water
Acid hydroxide → Ethanoate
CH3COOH + NaOH → CH3COOHNa + H2O
-
Acetic acid + sodium hydroxide → Sodium Acetate + Water
CH3COOH + NaOH → CH3COONa + H2O
-
Formic Acid + potassium hydroxide → Potassium methanoate + water
HCOH + KOH → HCOOK + H2
2. CARBOXYLIC ACID AND METAL (K, Na)
edu.uptymez.com
Acid + metal → Salt + hydrogen
Example
Acetic Acid + sodium → Sodium Acetate + Hydrogen
CH3COOH + Na → CH3COOHNa + H2
CH3COOH + K → CH3COOK + H2
ESTERIFICATION AND NEUTRALIZATION
ESTERIFICATION – Is the reaction between the Alcohol and carboxylic acid under control of concentrated sulphuric acid (H2SO4) and heat (Δ)
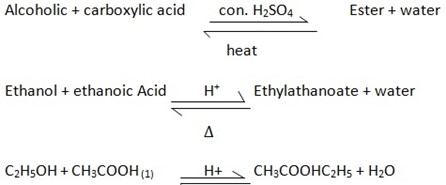
Acid provide – H- which shift equilibrium position than ester produced
CONDITION FOR ESTERIFICATION
They include
- Concentrated sulphuric acid (H2SO4) as catalyst. The concentrated sulphuric acid is used as the catalyst to speed up the rate of the chemical reaction.
- Heat.
edu.uptymez.com
PRODUCTS OF ESTERIFICATION
They include
- Ester
- water
edu.uptymez.com
ESTER – Are the organic compounds with the smell (sweet smell) like fruits and have structure
Example
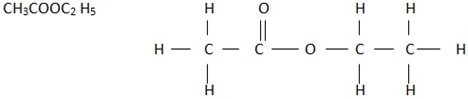
NEUTRALIZATION
This is the reaction between acid and base (Alkali) to form salt and water
Acid + Base → salt + water
-
Esterification and neutralization have differences as shown below.
DIFFERENCE BETWEEN ESTERIFICATION AND NEUTRALIZATION
edu.uptymez.com
|
|
ESTERIFICATION |
|
NEUTRALIZATION |
|
a) |
Is the reaction between carboxylic acid to form salt and water |
a) |
Is the reaction between acid and base Alcohol and form Ester and water |
|
b) |
Is reversible reaction |
b) |
Is not reversible (Irreversible) |
|
c |
Produce “Ester” |
|
Produce “salt” |
|
d) |
Catalyst used e.g. Acid |
d) |
catalyst is not used |
|
e) |
Produces covalent compound |
e) |
Produces the Ionic compound |
edu.uptymez.com
DIFFERENCES BETWEEN ALCOHOL AND CARBOXYLIC ACID
|
ALCOHOL – OH |
CARBOXYLIC ACID – COOH |
|
Are organic compound with hydroxyl group as functional group e.g. R – OH |
Are organic compound with carboxylic as group – COOH as functional group R – COOH |
|
Functional group not fixed |
Functional group is fixed |
edu.uptymez.com
SAPONIFICATIONS
Saponification is a process that produces soap, usually from fats and lye. It involves a reaction between a base, usually sodium hydroxide (caustic soda), and an ester group on a compound. Triglycerides are an example, which is an ester of a fatty acid. The triglycerides are hydrolyzed to form the sodium salt of a carboxylate. In addition to soap, such traditional saponification processes produce glycerol. “Saponifiable substances” are those that can be converted into soap
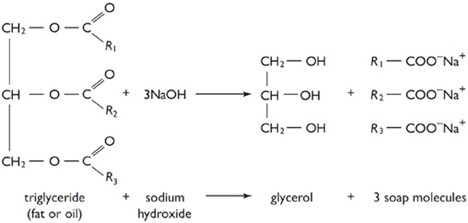
APPLICATION OF THE SAPONIFICATION
The knowledge of the saponification is applied in the industries to manufacture soap , Ester such as oil is mixed with the base then salt hence heated (boiled), then soap particles precipitates to form the down ward (boiled), then soap particles.