1. 1. Structure of the ATOM
-Describe the Rutherford and bohr’s models of the atom.
-Analyze atomic energy levels.
-Discuses the hydrogen energy levels, and derives expressions for the energy levels.
-Perform experiment to determine wavelength in the Balmer series of the hydrogen spectrum.
2. Quantum physics
– Describe failures of classical physics.
– Explain Planck’s quantum theory of blackbody radiation.
– Spectral nglish-swahili/distribution” target=”_blank”>distribution of black body radiation.
– Explain Einstein’s quantum theory of light.
– Perform experiment to determine the Planck’s constant.
– Account for the photoelectric effect phenomenon.
– Deduce stopping potential threshold frequency and work function of a metal.
– Explain the photo electric effect.
– Deduce de Broglie wave length for electron.
– Discus the wave- particle duality of electron.
– Derive de Broglie’s wavelength for the electron.
– Describe production and uses of x- rays.
– Uses in medicine, industry and in sample analysis.
3. 3. LASER
– Describe production of laser light.
– Explain properties of laser light.
– Distinguish types of lasers.
– Discuss methods of pumping in laser production.
– Identify application of laser light.
4. 4. Nuclear Physics
– Describe the structure of the nucleus.
* Review Rutherford experiment.
– Determine half life and the decay constant (λ) of a radioactive substance.
– Explain the relation of nuclear mass and binding energy.
* Discuss Einstein’s mass energy equation.
* Apply Einstein’s mass energy relation to determine the biding energy of nuclei.
– Identify criteria for stable and unstable nucleus.
* Analyze the neutron and proton ratio and plot N against Z for radioactive elements.
* Establish criteria for stable and unstable nuclei
– Identify uses and hazards of radioisotopes
* Application
* Hazards
– Distinguish between fission and fusion processes
* Meaning of fission and fusion
* Calculate the energy released in a nuclear fission
* Calculate the energy absorbed in nuclear fission
* Describe the application of nuclear fission and fusion
– Describe operation of a nuclease reactor
* Construction and operation of nucleus reactor for safe application
THOMSON’S MODEL OF ATOM
According to Thomson an atom is a positive charged sphere in which the entire mass and positive charge of the atom is uniform distributed with negative electrons embedded in it as shown.
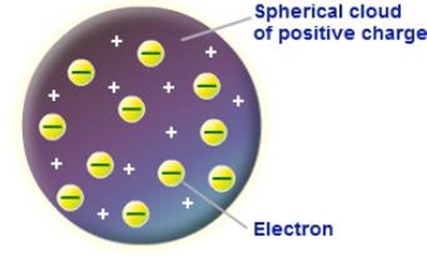
The number of electrons is such that their negative charge is equal to the positive charge of the atom. This atom is electrically neutral.
This model was called Thomson’s plum pudding model because the negatively charge electrons (the plums) were embedded in a sphere of uniform positive charge (the pudding).
Drawbacks of this Model
1.It could not provide stability to the atom it is because the positive and negative charges are stationary and will be drawn towards each other, thus destroying the individual negative and positive charges.
2. It could not explain the presence of discrete spectral lines emitted by hydrogen and other atoms.
RUTHER FORD’S MODEL OF ATOM
The salient features of this model are
(i)Every atom consist of a tiny central core, called the nucleus which contains all the atom’s positive charge and most of its mass (99.9%).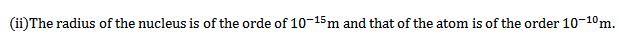

(iii) The electrons occupy the space outside the nucleus. Since an atom is electrically neutral the positive charge on the nucleus is equal to the negative charge on electrons surrounding the nucleus.
(iv) Electrons are not stationary but revolve around the nucleus in various circular orbits as do the planets around the sun.
In this way Rutherford provided stability to the atom. It is because the centripetal force required by the electrons for revolution is provided by the electrostatic force of attraction between electrons and the nucleus.
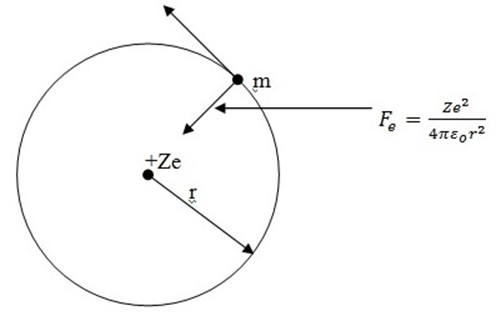
e = charge on electron
z =total number of protons in the nucleus
m=mass of the electron
r =distance of electron from the nucleus
v= linear velocity of the electron
Force of attraction between electron and the nucleus is
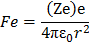
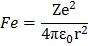
where Ze is a nuclear charge
The centripetal force required to keep the electron moving in circular path is
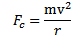
Since the atom is stable 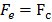
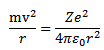
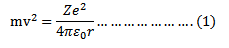
Kinetic energy of electron
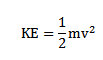
From equation (1)
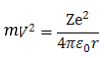
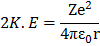
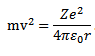
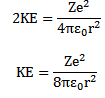
Potential energy of electron
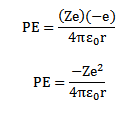
Total energy of electron
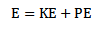
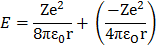
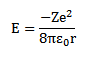
The total energy of electron in the orbit is negative hence the electron is bound to the positive nucleus
For hydrogen Atom
For hydrogen atom z= 1. Therefore K. E and P.E OF electron in hydrogen atom are
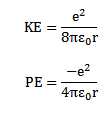
The total energy of electrons hydrogen atom is
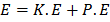
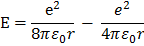
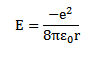
Limitations of Rutherford’s model of atom
1. According to Maxwell’s theory of electromagnetism a charge that is accelerating radiates energy as electromagnetic waves
The electron moving around the nucleus is under constant accelerating radiates energy as electromagnetic waves.
– Due to this continuous loss of energy the electrons in Rutherford’s model were bound to spiral towards the nucleus and fall into it when all of their rotational energy were radiated
– Hence Rutherford’s atomic model cannot be stable while in actual practice, an atom is stable
This shows that Rutherford’s model is not correct
1. During inward spiraling the electron’s angular frequency continuously increases
– As result electrons will radiate electromagnetic waves of all frequency i.e. the spectrum of these waves will be continuous in nature because these are continuous loss of energy.
– But this is contrary to observation experiments shows that an atom emits line spectra and each line corresponds to a particular frequency or wavelength.
Rutherford’s model failed to account for the stability of the atom. It was also unable to explain the emission of line spectra.
BOHR’S MODEL OF ATOM
According to Bohr’s atomic model, the revolving electrons in the atom do not emit radiations under all conditions. They do so under certain conditions as expalined by him in his model.
BASIC POSTULATES OF BOHR’S MODEL OF ATOM
1. The electrons revolve around the nucleus of the atom in circular orbits. The centripetal force required by electrons for revolution is provided by the electrostatic force of attraction between the electrons and the nucleus.
2. An electron can revolve only in those circular orbits in which its angular momentum is an integral multiple of 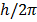
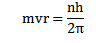
h= Plank’s constant.
Radius of orbit r
From,
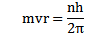
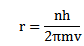
Since n is a whole number only certain value of r is allowed.
Thus according to Bohr, an electron can revolve only in certain orbits of definite radii not in all these are called stable orbits (stationary orbit)
According to this postulate the angular momentum of the electron does not have continuous range i.e. the angular momentum of the revolving electron is quantized.
While revolving in stable or stationary orbits the electrons do not radiate energy inspite of their acceleration towards the centre of the orbit.
– For this reason these permitted orbits are called stable or stationary orbits.
e= charge on electron
m= mass of electron
rn= radius of the nth orbit
vn= velocity of electron in the nth orbit
Z= number of positive charge (protons)
Positive charge on nucleus Ze
RADIUS OF BOHR’S STATIONARY ORBITS
As the centripetal force is provided by the electrostatic force of attraction between the nucleus and electron.
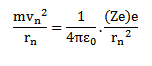
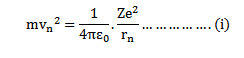
According to Bohr
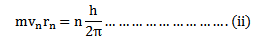
Consider equation 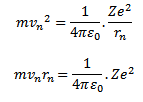
Take equation (ii) square it
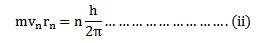
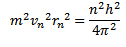
Take equation (iii) 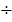 equation (i)
equation (i)
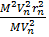 =
= 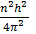 .
. 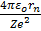
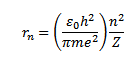
It is clear that 
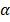 n2, radii of the stationary orbits are in ratio 12: 22:32 ………..clearly the stationary orbits are not equally spaced.
n2, radii of the stationary orbits are in ratio 12: 22:32 ………..clearly the stationary orbits are not equally spaced.
For hydrogen atom
For hydrogen atom z= 1, so that equation become 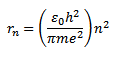
Now 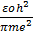 = 0.53 x10-10m
= 0.53 x10-10m
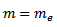
e = electronic charge 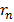 = (0. 53 x 10-10)
= (0. 53 x 10-10)
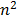 metres
metres
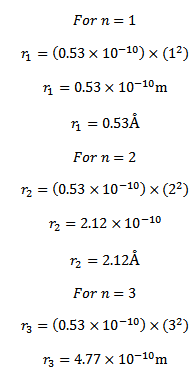
Thus the radii of the first, second and third stationary orbits of hydrogen atom are 0.53 Å, 2.12 Å and 4.77Å respectively.
2. VELOCITY OF ELECTRON IN BOHR’S STATIONARY ORBIT
From equation below, we have
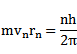
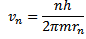
Putting the value of 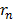 into that equation
into that equation
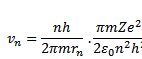
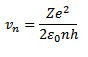
It is clear that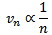 in other words, electrons move at a lower speed in higher orbits and vice versa.
in other words, electrons move at a lower speed in higher orbits and vice versa.
For hydrogen atom
Z =1
Then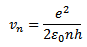
3. FREQUENCY OF ELECTRON IN STATIONARY ORBIT
The number of revolution completed per second by the electron in a stationary orbit around the nucleus
Velocity of electron in the 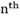 orbit
orbit
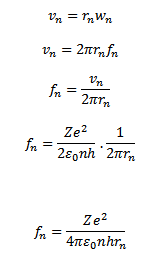
For hydrogen atom
Z = 1
Then,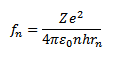
Frequency of electron in the first orbit of hydrogen atom is n=1, r1=0.53×10-10m
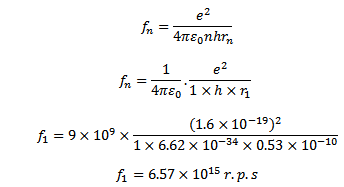
Electron in first orbit of hydrogen atom will have a frequency of 6.57x 1015revolutions per second.
4. TOTAL ENERGY OF ELECTRON IN STATIONARY ORBIT
The total energy En of the electron in the nth orbit is the sum of kinetic and potential energy in the nth orbit.
– The K.E of electron in the nth orbit is
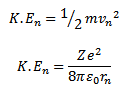
The potential energy of electron in the nth orbit is
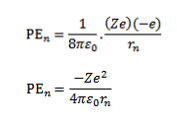
Total energy of electron in the nth orbit is
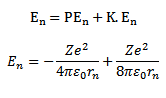
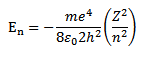
But
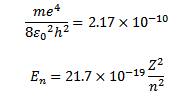
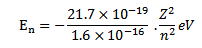
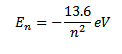
Thus as n increases i.e. electron moves to higher orbit, the total energy of the electron increases i.e. total energy becomes less negative.
For hydrogen atom z=1
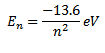
Thus the total energy of electron in a stationary orbit is negative which means that the electron is bound to the nucleus and it is not free to leave the atom.
We can find the total energy of electron in the various orbits of hydrogen atoms as under.
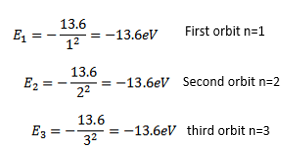
The total energy of electron increases i.e. becomes less negative as the electron goes to higher orbits
When n→∞ En =0 and the electron becomes free
Ground state/ normal state
This is the state of atom when the entire electrons in it occupies their lowest energy levels as required by their n and l values.
The energy of an atom is least i.e. largest negative value when n=1 i.e. when electron revolves in the first orbit.
The energy of hydrogen atom in the ground state is 13.6eV.
Excited state
This is the state of an atom when electrons in an atom occupy energy levels higher than those permitted by the values of n and l values.
At room temperature most of the hydrogen atoms are in the ground state
If hydrogen atom absorbs energy i.e. due to rise in temperature it may be promoted to one of the higher orbits (i.e. n=2, 3, 4…..)
The atom is said to be in the excited state.
WAVE LENGTH
OF EMITTED
RADIATION.
When an electron jumps from a higher orbit (n2) to the lower orbit (n1) the energy difference between the two orbits is released because the energy of electron in the higher orbit is more than in the lower orbit. Consider two orbits having principle quantum numbers n2 and n1 where n2>n1
Then energy of electron in the two orbits is given by
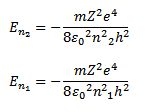
As the electron jumps from orbit n2 to n1, energy is released in the form of electromagnetic radiation.
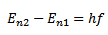
where
f= frequency of the emitted radiation
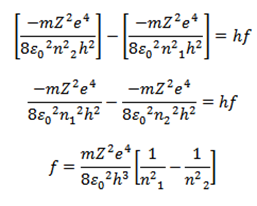
The wavelength of the emitted radiation is given by
c=λf
 =
= 
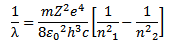
This equation gives the wavelength of emitted radiation.
Now,
 =
=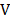 = wave number
= wave number
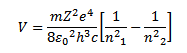
Wave number
These are the number of waves in a unit length.
For hydrogen atom
For hydrogen atom z = 1
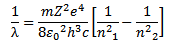
This gives the mathematical formula for the wavelength of radiation emitted by hydrogen atom when electron jumps from outer orbit to inner orbit.
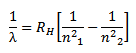
where
RH is Rydberg constant. The value of RH can be calculated as the value of e, m, h and c are known
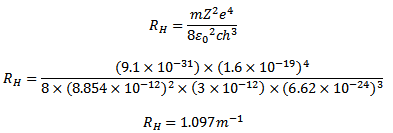
HOW TO CALCULATE THE RYDBERG CONSTANT USING CALCULATOR
From
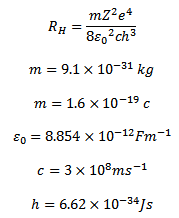
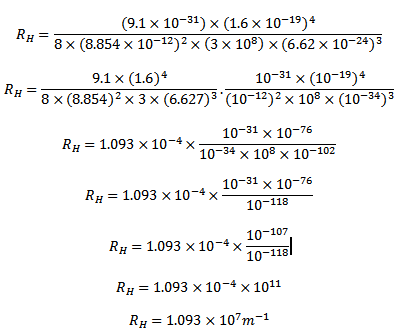
Clearly, wavelength/frequency of radiation emitted from the excited atom is not continuous. They have definite value depending upon the values of 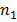 , and
, and 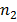
SPECTRAL SERIES OF HYDROGEN ATOM
Bohr gave a mathematical explanation for the spectrum of hydrogen atom.
The whole hydrogen spectrum can be divided into district groups of lines each group of lines is called spectral series.
The wavelength of the lines in each group can be calculated from Bohr’s formula
 =
=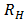
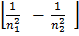
The following are spectral series of hydrogen atom
i) Lyman series
ii) Balmer series
iii) Paschen series
iv) Bracket series
v) Pfund series
i) Lyman series
The Lyman series is obtained when electron jump to first orbit n1=1 from outer orbits (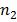 =2, 3, 4…)
=2, 3, 4…)
Therefore the formula for calculating the wavelength of the lines in this series is,
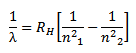
where
This series lies in the ultraviolet region which is the invisible region.
ii) Balmer series
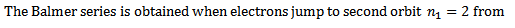
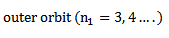
Therefore the formula for calculating the wavelength of the lines in this series is 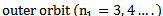
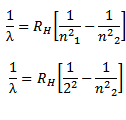
where
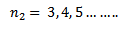
This series lies in the visible spectrum and was found first of all in the hydrogen series
iii) Paschen series

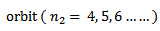
Therefore the formula for calculating the wavelength of the lines in this series is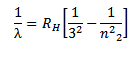
where
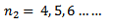
This series lies in the infrared region.
iv) Brackett series
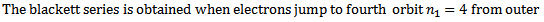
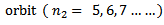
Therefore the formula for calculated the wavelength of the lines in this series is
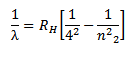
This series lies in the infrared region.
v) Pfund series
The Pfunds series is obtained when electrons jump to fifth orbit n1 = 5 from outer orbits (n2
=6, 7, 8…..)
Therefore the formula for calculating the wavelength of the lines in this series is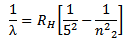
where
(n2
=6, 7, 8…..)
This series also lies in the infrared region
ENERGY LEVEL DIAGRAM
Energy level diagram is a diagram in which the total energies of electron in different stationary orbit of an atom represented by parallel horizontal lines drawn according to some suitable energy scale
In order to draw energy level diagram of an atom we must know the total energy of electron in different stationary orbits.
The total energy of an electron in the nth orbit of hydrogen atom is given by
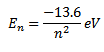
By putting value of n=1, 2, 3….. we can find the total energy of electron in various stationary orbits of hydrogen atom as
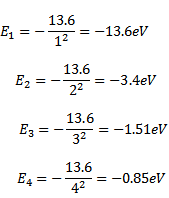
Similarly we can find the total energy of electron in the higher orbits
The table below gives the total energy of electron of hydrogen atom in different stationary orbits.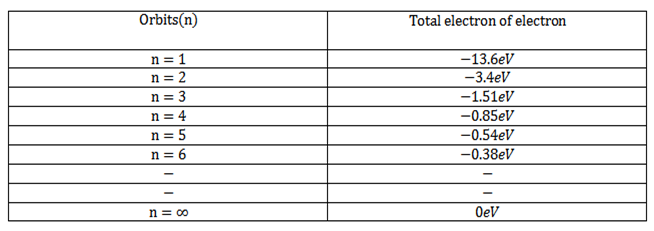
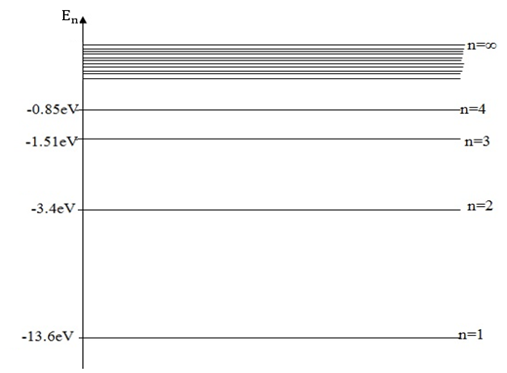 The energy level diagram of hydrogen atom is shown below
The energy level diagram of hydrogen atom is shown below
Total energy of electron in a stationary orbit is represented by a horizontal line drawn to some suitable energy scale.
(i) The hydrogen atom has only one electron and this normally occupies the lowest level and has energy of -13.6eV
When the electron is in this level the atom is said to be in the ground state.At room temperature nearly all the atoms of hydrogen are in ground.
(ii) If hydrogen atom absorbs energy (due to rise in temperature )the electron may be promoted into one of the higher energy levels
The atom is now said to be in an excited state.Thus when the electron occupies other than the lowest energy level the atom is said to be in the excited state.
(iii) Once in an excited state the atom is unstable after a short time interval the electron falls back into the lowest state so that the atom is again in the ground state.
The energy that was originally impacted is emitted as electromagnetic waves.
(iv) The total energy of electron for (n= ) it becomes free of atom.
) it becomes free of atom.
The minimum energy required to free the electron from the ground state of an atom is called ionization energy
For hydrogen atom ionization energy is +13. 6eV
(v) The difference between the adjacent energy goes on decreasing as the value of n increases.
So much so that when n>10 the energy difference is almost zero this is show by closeness of energy level lines at higher levels.
(vi) Note that region is labeled continuous at energy above zero n=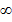 level, the electron is free from the atom and is at rest
level, the electron is free from the atom and is at rest
Higher energy represents the translation kinetic energy of the free electron
This energy is not quantized and so all energies above n =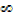 are allowed
are allowed
IMPORTANT TERMS
It is desirable to discuss some important terms much used in the study of structure of atom.
(i) EXCITATION ENERGY
Excitation energy is the minimum energy required to excite an atom in the ground state to one of the higher stationary state.
Hydrogen atoms are usually in their lowest energy state where n=1
In this state (ground state) they are said to be unexcited.
However if you bombard the atoms with particles such as electron or proto collision can excite them
In other words a collision may give an atom enough energy to change it from ground state to some higher stationary state.Consider the case of hydrogen atom we know that 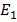 = -13.6eV (ground state
= -13.6eV (ground state 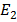 = -3.4eV (first excited state)
= -3.4eV (first excited state) 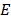 =1.51eV (second excited state) and
=1.51eV (second excited state) and 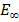 =0
=0
In order to lift an electron from ground state n =1 to the first excited state n=2 energy required is E
E =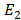 –
– 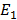
E= -3.4 – (- 13.6)
E = 10.2eV
Therefore the bombarding particle must provide an energy of 10.2eV to excite the atom from n =1 state to n=2 state
Similarly to excite the atom from n=1 state to n=3 state energy required is
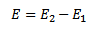
E = – 1.51 – (-13.6)
E = 12.1eV
We say that first and second excitation energies of hydrogen are 10.2eV and 12.1eV respectively
(ii) EXCITATION POTENTIAL
Excitation potential is the minimum accelerating potential which provide an electron energy sufficient to jump from the ground state n=1 to one of the outer orbits
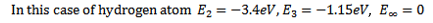
Energy required to lift a electron from ground state n=1 to n=2 state is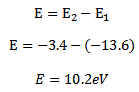
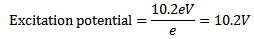
Hence excitation potential for the first excited state of hydrogen is 10. 2V
Similarly energy required to lift an electron from ground state n=1 to n=2
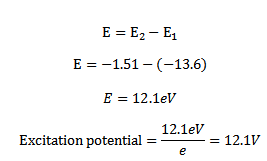
The value of excitation potential depend upon the state to which the atom is excited to which the atom is excited from the ground state
(iii) IONIZATION ENERGY
Ionization energy is the minimum energy needed to ionized an atom
Consider the case of hydrogen atom it has only one electron and this normally occupies the ground state.
The energy of the electron for n=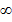 state is zero and if the electron is lifted to this level (n=
state is zero and if the electron is lifted to this level (n=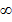 ) it becomes free of hydrogen atom i.e. hydrogen atom is ionized
) it becomes free of hydrogen atom i.e. hydrogen atom is ionized
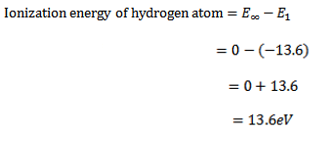
(iv) IONIZATION POTENTIAL
Ionization potential is the minimum accelerating potential which would provide electron energy sufficient to just remove the electron from the atom.
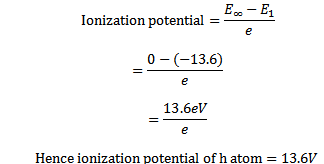
The ionization potential of one electron atom or ion is given by

(v) QUANTIZATION OF ENERGY
Quantization of energy is the existence of energy radiated by atoms in a specific amount which is are integral multiples of a constant (hf).
SUCCESS OF BOHR’S THEORY
The success of bohr’s theory is not to be attributed so much to the mechanical picture of atom he proposed but rather to the development of mathematical explanation that agrees exactly with experimental observations. Bohr’s theory achieved the following successes.
i) MADE ATOM STABLE
Bohr’s theory made the atom stable according to this theory an electron moving in the formatted (quantum) orbits cannot lose energy even though under constant acceleration. This provided stability to the atom.
ii) INTRODUCED QUANTUM MECHANICS
Bohr’s theory introduced quantum mechanics in the realm of atom for the first time
Bohr’s explained that sub- atomic particles e.g. electrons are governed by the laws of quantum mechanics and not by classical laws of electron hydrogen as assumed by Rutherford
This completely changed our thinking and was the major step towards the discovery of the rudiment laws of the atomic world
iii) GAVE MATHEMATICAL EXPLANATION OF HYDROGEN SERIES
The hydrogen series found by various scientists were based on empirical relation but had no mathematical explanation
However these relations were easy derived by applying Bohr Theory
Further the size of hydrogen atom as calculated from this theory agreed very closely with the experimental value.
LIMITATIONS OF BOHR’S THEORY
Bohr’s simple theory of circular orbits inspire of its many successes was found inadequate to explain many phenomena observed experimentally.
This theory suffered from the following drawbacks.
(i) It could not explain the difference in the intensities of emitted radiations.
(ii) It is silent about the wave properties of electron
(iii) It could not explain experimentally observed phenomena such as Zeeman Effect, Stack effect etc.
(iv) Bohr’s model does not explain why the orbit are circular while elliptical path is also possible
(v) It could only partially explain hydrogen atom. For example this theory does not explain the fine structure of spectral lines in the hydrogen atom