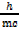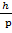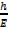THE QUANTUM THEORY
WAVE PARTICLE DUALITY NATURE OF MATTER
States that: “Matter has particle nature as well as wave nature”. This means that matter have dual properties or two properties such as particle nature and wave nature. The wave particle duality nature of matter was put forward by De Broglie scientist. De Broglie’s derived an expression which applied to find the De Broglie’s wave length.
De Broglie’s wave length is in terms of mass and momentum.
Hence from Einstein equation;
E = mc2 …………………….(i)
From Planck’s equation
E = 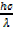 ……………… (ii)
……………… (ii)
Compare equation (i) and (ii)
mc2 = 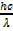
De Broglie’s wave length in terms of energy
From:
λ = 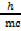 multiply by 1/c in both side
multiply by 1/c in both side
λ  =
= 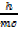 [1/c]
[1/c]
 =
= 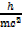
Since E = mc2
Example
Alpha particles emitted from radium have energy of 4.4MeV. What is the de-Broglie’s wave length?
The mass of moving particles is 9.01 x 10-19g. What is the de-Broglie’s wave length?
The momentum of particles is 2.0 x 10-10gm/s. What is the de-Broglie’s wave length?
Given that:
h = 6.63 x 10-34 JS
C = 3.0 x 108m/s
Solution
a. E = 4.4MeV
λ =?
De Broglie’s
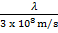 =
= 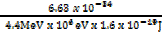
λ = 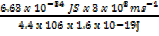
λ = 4.52 x 10-13m
b. Solution
m = 9.01 x 10-19g
λ =?
De Broglie’s
λ = 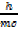
λ = 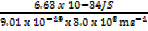
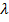 = 2.33 x 10-24m
= 2.33 x 10-24m
c. P = 2.0 x 10-10gms
λ =? De Broglie’s
λ = 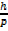
λ = 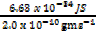
λ = 3.315 x 10-24Jg-1m-1s-1