RULES FOR FILLING ELECTRONS IN THE ORBITALS OF AN ATOM
The orbitals of an atom can be filled with electrons by applying the following rules.
i)Aufbau Principle
This is also known as the building up principle. According to this principle “The electrons in an atom are so arranged that they occupy orbitals in the order of their increasing energy.”
Thus, the orbital with the lowest energy will be filled first, then the next higher in energy, and so on. Since, the energy of an orbital in the absence of any magnetic field depends upon the principal quantum number (n) and the azimuthal quantum number (l), hence the order of filling orbitals with electrons may be obtained from the following generalizations.
a) The orbitals for which n + I is the lowest is filled first.
b) When two orbitals have the same values of n+l, the orbital having the lower value of n is filled first.
The order of filling of various orbitals with electrons obtained by this rule is given below:
1s, 2s. 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f,5d, 6p, 7s, 5f…
To remember this sequence may be a difficult task. Given a long side is a simple way of working out this order. In this method a series of arrows running from upper right to the lower left gives the order of orbitals with increasing energy.
ii) Pauli’s Exclusion principle
In 1925 Wolfgang Pauli discovered what is known as the exclusion principle. This principle is very useful in constructing the electronic configuration of atoms. According to this principle “No two electrons in an atom can have the same values for all the four quantum numbers”.
For example in 1s orbital of helium atom there are two electrons. According to the concept of quantum number and Pauli’s rule, their quantum numbers are:
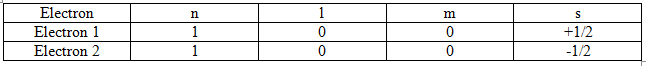
The + and – sign before j refers to the clockwise and anticlockwise spins of the electrons.
Thus, the two electrons having the same values of n, I and m could have different values
of s, i.e., their spins are in the opposite directions. This leads to a very significant observation that
“Each orbital can accommodate at the maximum two electrons with opposite spins.”
Applications of the Pauli’s exclusion principles
The Pauli’s exclusion principle leads to the following conclusions:
(a) An orbital cannot have more than two electrons.
(b) In any main energy level (shell), the maximum number of electrons is twice the in number of orbitals, or is equal to 2n2, where n is the principal quantum number.
iii) Hund’s Rule of Maximum Multiplicity
The rules discussed above do not give any idea for filling electrons into the orbitals having equal energies (such states are called degenerate states). For example, three p-orbitals, i.e. px, py and pz, have equal energy. How should electrons be filled into these orbitals? Let us take an example in which three electrons are to be filled into three p-orbitals. The three electrons can be filled into three p orbitals in two different ways as shown below.
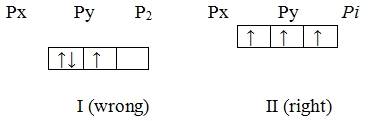
Now, which of the two is correct? The answer to this question is given by Hund’s rule, Hund’s rule states that,
“When more than one orbitals of equal energies are available, then the electron*,] first occupy these orbitals separately with parallel spins. The pairing of electrons will | only after all the orbitals of a given sub-level are singly occupied.”
According to the Hund’s rule, the correct way of filling three electrons in three p orbitals that in which each orbital is singly occupied, (arrangement II above).
Hund’s rule is also known as the Hund’s rule of maximum multiplicity.
Explanation
Two electrons with parallel spins, tend to be as far apart as possible to minimize the electrostatic repulsion. Therefore, the electrons prefer to occupy the orbitals singly
as far as possible. When all the orbitals get singly occupied, then the incoming electron has two choices either to pair up with the other electron or to go to the next higher orbital.
When vacant orbital of suitable energy is not available, then the incoming electron will have no choice except to pair up with another electron.
Example
(a) State the;
(i) Heisenberg uncertainty principle.
(ii) Hund’s rule.
(iii) Paul’s Principle.
(iv) Aufbau’s Principle.
(b) Brief explain why the following sets of quantum number are NOT allowed in hydrogen atom:
(i) n = 1, l = 1, ml = 0
(ii) n = 1, 1 = 0, ml = 2
(iii) n = 4,1 = 3, ml = 4
(iv) n = 0, 1 = 0, ml = 0 and
(v) n = 2, 1 = –1, ml = 1
(c) How many orbitals are there in each of the following sublevel?
(i) 1s
(ii) 2p
(iii) 3d
(iv) 4f
(d) How many sublevels are there in each of the following shells?
(i) K
(ii) N
(iii) L
(e) Which of the following electronic configuration is correct for 14p in its ground state?
State the principle violated in each case
Solution
(a) (i) The Heisenberg’s uncertainly principle states that; “it is impossible to measure simultaneously the exact position and exact velocity (momentum) of an electron.” Any attempt to measure one quality will distract the measurements of the other quantity
(ii) Hund’s rule states that, “electron pairing is not allowed until all orbitals of the particular energy sub – level are occupied by at least one electron.”
(iii) Paul’s Exclusion Principle, “states that no two electrons may have all the four quantum number the same.” Two electrons may have three quantum numbers of the same; the paired electrons are always in opposite direction at the same time.
(E.g.  and
and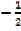 ).
).
(iv)Aufbau Principle states that, “In electrons configuration, electrons are filled in order of increasing energy levels.” Thus the lowest energy available must be filled up first. The orbital energy increase in the following order; is 2s, 2p, 3s, 4p, 5p etc.
(b) (i) n = 1, 1 = 1, ml = 0 this is not allowed because l must be smaller than n, in the case l = 1 which is not allowed.
(ii) n = 1, 1 = 0, ml = 2. This is not allowed because 1 = 0 and ml = 0
(iii) n = 4, 1 = 3, ml = 2. This is not allowed because for 1 = 3 can range from –3, +3, thus, +4 is not allowed.
(iv) n = 0, l = 0, ml = 0; this is not allowed because n cannot be zero.
(v) n = 2, l = -1, ml = 1. This is not allowed because l cannot be a negative number and n can never be = 0
(c) (i) 1 s sub energy level has one – orbital (which is 1 s – orbital).
(ii) 2 p sub energy has three p – orbital (which is 1 s – orbital).
(iii) 3 d sub energy level has five d – orbital [which are 3 d (x2 – y2), 3dz2 3dxy, 3 and 3dyz].
(iv) 4 f sub energy level has seven f – orbital [which are 4xy, 4fyz, 4fz, 4f(x2 – y2),
4f(x2-z2) and 4f (y2 – z2)].
(d) (i) K has one sub level (which is l s2).
(ii) N has four sub levels (which are 4s2, 4p6, 4d10 and 4f14).
(iii) L has two sub levels (which are 2s2 and 2p6).
(e) Is the correct electronic configuration of 15p
This is correct since it obeys principle governing electron in an atom nglish-swahili/distribution” target=”_blank”>distribution
(i) [(Ne)]
(ii) [(Ne)] aufbau (building up) principle is violated as it states that electrons available as the case here.
(iii) [(Ne)]
(iv) [(Ne)] Hund’s Rule of maximum that multiplicity is violated as it states electrons occupy orbital as singly as possible
Example
(a) State the rules used in filling electrons in various orbitals of an atom.
(b) By using 3 different ways for each of the following atoms write their electron configuration
(i) C
(ii) N
(iii) Ag
(c)Write the electronic configuration of Na+ and F– then show the other element whose electronic configuration resembles these ions. Show that electronic configurations of Cu and Cr are unusually written. Give reasons.
Solution
(a)(i)Paul’s exclusion principle states that; “no two electrons may have all the four quantum number the same i.e. electrons may occupy the same orbital only on conditions that they have their spins in the opposite direction.”
(ii) Hund’s rule of maximum multiplicity states that, “when more than one orbital with equal energies are available, electrons tend occupy those orbital separately first with parallel spins and separately first with parallel spin and pairing of electrons will start only after all the orbitals of a given sub level are singly occupied.”
(iii) Aufbau principle states that, “Electrons in an atom are so arranged that they occupy the orbitals in the order of their increasing energy.”
(b) (i) C Atomic number 6
Using Box method:
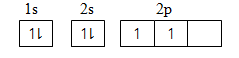
1s2 2s2 2p2
Using noble gas structure;
[He] 2p2
(ii) N atomic number 7
Using box method

Using sub levels only;
1s2 2s2 2p3
Using noble gas structure;
[He] 2p3
(iii) Ag atomic number 47
Using box method;
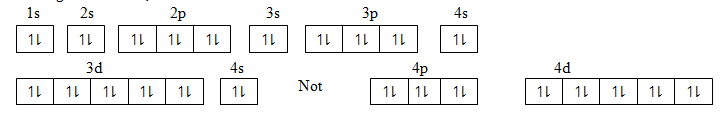
Note: 4d is filled first to maintain stability of full filled d – orbital before 5s is filled.
Using sub levels only;
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 4d10 5s1
Using noble gas structure;
[Kr] 4d10 5s1
(c)
(i) Na+ number of electrons also 10
1s2 2s2 2p6 resembles Ne
(ii) F – number of electrons also 10
1s2 2s2 2p6 resembling Ne
(d) Cu Atomic number 29
e.g. [Ar] 4s1 3d10
Cr Atomic number 24
e.g. [Ar] 4s1 3d5 full filled and half filled orbitals are very stable electronic structures. The stability gained is the cause of 4s electrons to be unpaired and make 3d orbitals either full filled or half filled.
Note that: In writing the electronic configuration the atoms or orbitals of the same value are usually written together irrespective of their relation energy levels.
E.g. Cu 1s2 2s2 2p6 3s2 3p6 3d10 4s1 not 1s2 2s2 2p6 3s2 2p6 4s1 3d10. To save time and space noble gases are often utilized e.g. sodium [Ne] 3s1 and Iron [Ar] 3d6 4s2