CHEMICAL BONDING AND MOLECULAR STRUCTURE
Definition:-chemical bond is the chemical force /force of attraction which keeps the atoms in any molecule together.
Types of the chemical bond
i) Ionic/Electrovalent bond
ii) Covalent bond
iii) Coordinate bond
iv) Polar covalent bond
v) Non-polar covalent bond
vi) Hydrogen-bonding
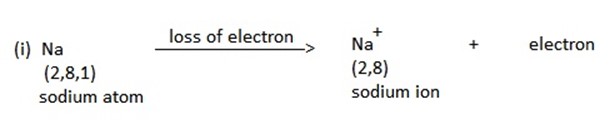
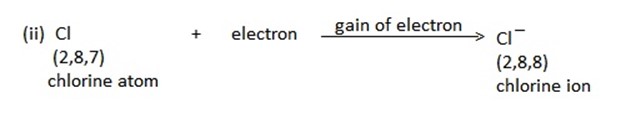
i) IONIC/ ELECTROVALENT BOND.
An ionic or electrovalent bond is formed by complete transfer of one or more electrons from the atom of the metal to that of a non-metal.
Note: – As a result of electron transfer the following changes occur in the reacting atom:-
(a) Both the atoms acquire stable noble gas configurations.
(b) The atoms that loses its electrons becomes positively charged ion called cation, the atom which gain this electrons becomes negatively charged ions called anion.
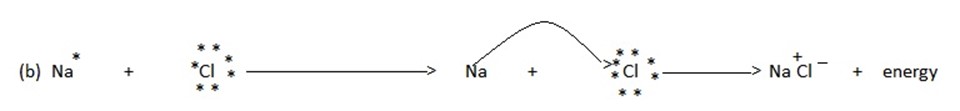
(c) The two oppositely charged ions .i.e. the cation and the anion are then held together by the coulombic force of attraction to form an ionic bond .
Note:- During the formation of ionic bond, a certain amount of energy is released.
e.g Na + Cl → Na+cl–
This ionic bond may be defined as the coulombic force of attraction which holds the oppositely charged ions together.
ELECTROVALENCY
The number of electrons lost or gained by an atom of any element is termed as electrovalency. The element which gives up the electron(s) to form positive charge or ions are said to have positive valency. While the element(s) which accepts electrons to form negative ions are said to exhibit negatively valency.
Note:- Variable electrovalency of iron exist as Fe2+ and Fe3+ in ferrous sulphate and ferric sulphate .when a compound is formed by the transfer of electrons, the element that loses electron(s) is said to be oxidized and the element that gains electrons is said to be reduced.
Oxidation is a process which involves loss of electrons where as reduction is the process which involves gaining of electrons.
PROPERTIES OF IONIC/ELECTROVALENT BOND
An ionic/Electrovalent bond has the following properties:-
(i) An ionic bond is formed due to the coulombic attraction between the positively and negatively charged ions.
(ii) An ionic bond is non-directional, the strength of interaction between two ions depend upon distance but not the directional.
(iii) An ionic bond gets broken when the substance is dissolved in polar solvent such as water or when the substance is melted.
Typical examples of ionic bond:-
(a) Na + Cl → NaCl
FACTORS INFLUENCING THE FORMATION OF IONIC BOND
The main steps involved in the formation of an electrovalent/ ionic bond are:-
(i) Removal of electrons from one atom, in this stage energy equal to the ionization energy is absorbed.
(ii) Gaining of electrons by the other atoms .In this step energy equal to the electrons affinity is released.
(iii) Combination of cation and anions. These ions are held together by coulombic force of attraction. In this step, energy equal to the lattice energy is released.
(II) COVALENT BOND
A covalent bond is formed between two atoms (similar and dissimilar) by a mutual sharing of electrons .the shared pairs of electrons are counted towards the stability of both the participating atoms.
DEFINITION: – A covalent bond is defined as the force of attraction arising due to mutual sharing of electrons between the two atoms.
When the two atoms combine by mutual sharing of electrons, then each of the atoms acquire stable configuration of the nearest noble gas.
COVALENCY
Is the number of electrons which an atom contributes towards mutual sharing during the formation of a chemical bond.
Example of the Covalency: – Covalency of hydrogen (H2)
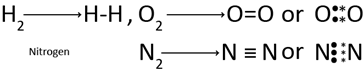
CHARACTERISTICS OF COVALENT BOND
(i) Mode of formation, covalent bond are formed due to mutual sharing of one or more pairs of electrons.
(ii) Directional character , covalent bonds are directional in nature this is because the shared electrons remains localized in a definite space between the nuclei of the two atoms.This gives a directional character to the covalent bond.
SINGLE COVALENT BOND
A covalent bond formed by mutual sharing of one pair of electrons. A single covalent bond is represented by a small line (-) between the two atoms.
E.g
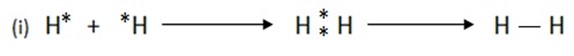
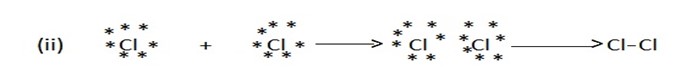
MULTIPLE COVALENT BONDS
The covalent bonds developed due to mutual sharing of more than one pairs of electrons are termed as multiple covalent bond.
The multiple covalent bonds are:-
(i) Double covalent bond
(ii) Triple covalent bond
Double covalent bond is the bond formed between two atoms due to the sharing of two electrons pairs. Its simply called Double bond
E.g. O2 → O = O
CO → C = O etc.
Triple covalent bond is the bond formed due to the sharing of three electron pairs
E.g N2 → N ≡ N,
Acetylene H – C ≡ C – H.
(I) FORMATION OF MOLECULES HAVING DOUBLE BOND.
(i) Formation of oxygen (O2) molecule:-
Each oxygen atom has six electrons in its outer most shell. Thus it requires two more electrons to achieve the nearest noble gas configuration.
E.g
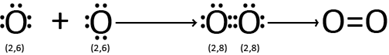
(ii) Formation of carbon dioxide(CO2) gas
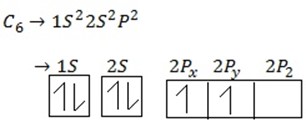
The electronic configuration of carbon and oxygen are :-
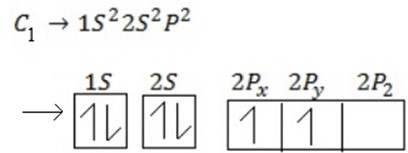
C 1s2 2s22p2 2,4
O 1s2 2s22p4 2,6
Thus each carbon atom requires four ,and each oxygen atom requires two more electrons to acquire noble gas configuration.This is achieved as follows
e.g

(iii) Formation of molecules having triple bond :-
(a) Formation of nitrogen (N2) molecule.
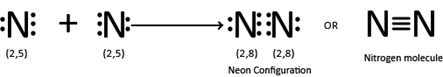
COMPARISON BETWEEN SINGLE,DOUBLE AND TRIPLE COVALENT BONDS
Triple bond length < Double bond length < Single bond length.
Since a shorter bond means greater bond strength hence the energy required to separate the bonded atoms( called bond energy) follows the order
Triple bond > Double bond > Single bond
FACTORS FAVOURING THE FORMATION OF COVALENT BOND
The following are the factors that favour the formation of a covalent bond:-
(i) High ionization enthalpy (energy).
The element having higher ionisation enthalpy (energy) cannot lose electrons easily.
(ii) Nearly equal electron gain enthalpy or electron Affinity.
The atoms of the two elements which have equal or nearly equal electron gain enthalpies or electrons affinities tend to complete their outer shells by mutual sharing of electrons.
(iii) Nearly equal electronegativity.
Equal or nearly equal electronegativity of the two combining elements does not permit the transfer of electron(s) from one atom to another.
(iv) High nuclear charge and small atomic size.
High nuclear charge, and smaller atomic size of the combining elements favour covalent bond formation, because the transfer of electrons in such case will not be possible.
COORDINATE COVALENT BOND
Coordinate bond is formed when the shared electron pair is provided by one of the combining atoms.The atom which provides the electron pair is termed as the donor atom ,while the other atoms which accept is termed as the acceptor atom
The bond formed when one sided sharing of electrons take place is called a coordinate or Dative bond. A coordinate bond is presented by an arrow ( → ) pointing towards the acceptor atom.
(i) Formation of coordinate bond during the formation of a molecule or molecular ion:-
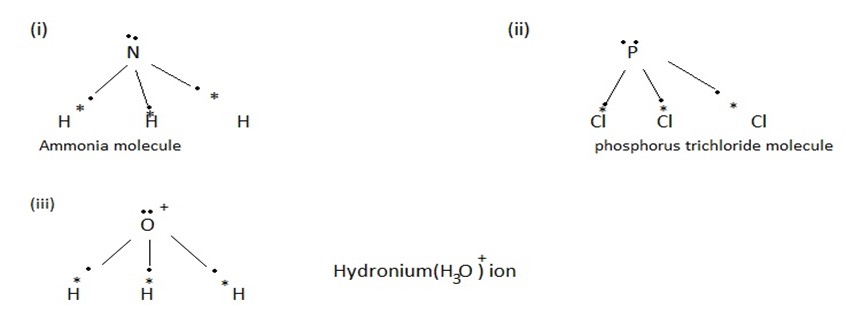
(a) Formation of Ammonium (NH4+) ion.
During the formation of ammonium ion ,nitrogen is the donor atom while H+ is the acceptor ion.
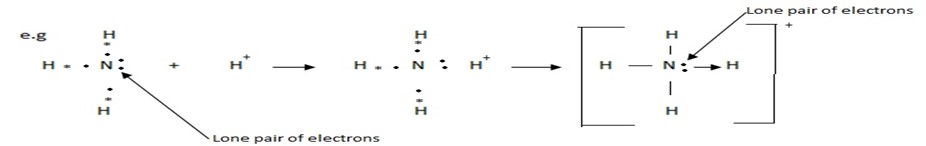
(b) Formation of coordinate bond between two molecules:-
Two or more stable molecules combine to form a molecular complex,is such a complex molecule, the constituent molecules are held together by coordinate bond
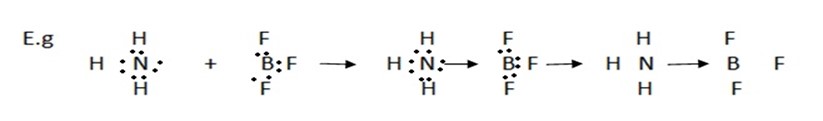
The product above is ammonia-borontrifluoride complex
POLARITY IN COVALENT BONDS :-
Depend upon chemical nature of the combining elements, the following two types of covalent bond are formed
(i) Non-polar covalent bond
(ii) Polar covalent bond
(i) NON-POLAR COVALENT BOND
When a covalent bond is formed between two atoms of the same element, the electrons are shared equally between the two atoms .The resulting molecule will be electrically symmetrical.
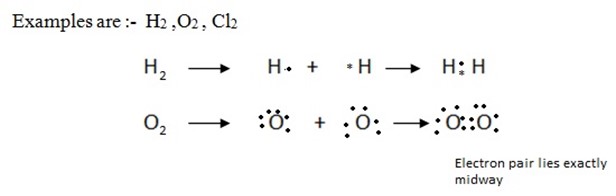
(ii) POLAR COVALENT BOND
When a covalent bond is formed between two atoms of different elements, the bonding pair of electrons does not lie exactly midway between the two atoms. It lies more towards the atom which has more affinity for electrons.
The atom with higher affinity for electrons thus develops a slight negative charge and the atom with lesser affinity for electrons a slight positive charge, such molecules are called polar molecules.The covalent bond between two unlike atoms which differ in the affinities for electrons is said to be a polar covalent bond.
Example of polar covalent bond are hydrogen chloride (H Cl) , water (H2O)
CAUSES OF POLARITY IN BONDS
The cause of polarity in bond is due to the electronegativity difference.
Example of the electronegativity difference between hydrogen and halogen atoms is as follows:-
halogen atoms: (F,Cl,Br,I)
electronegativity differences:-
H – F > H – Cl > H – Br > H – I
(4- 2.1) (3.0- 2.1) (2.8- 2.1) (2.5- 2.1)
1.9 0.9 0.7 0.4
VALENCE SHELL ELECTRON PAIR REPULSION (VSEPR) THEORY.
The VSEPR theory was proposed by R.J Gillespie and R.S Nyholmn in 1957. This theory was developed to predict the shapes of the molecule in which the atom are bonded together with single bond only. This theory is based on the repulsions between the electron pairs in the valence shell of the atoms in the molecule. The main postulate of the vsepr theory are:-
(i) The geometry of a molecule is determined by the total number of electron pairs (bonding and non-bonding) around the central atom of the molecule.
The shape of the molecule depends upon the orientation of these electron pairs in the space around the central atom.
(ii) The electron pairs (shared or lone pairs) around the central atom in a molecule tend to stay as far away from each other as possible so as to minimize the repulsion forces
between them.
(iii) The strength of repulsion between different electron pairs follows the order.
Lone pair-lone pair > lone pair-shared pair > shared pair-shared pair
The shared pair of electrons are also called bond pair of electrons.The presence of lone pair(s) of electrons on the central atom causes some distortions in the expected regular shape of the molecules.
PREDICTING THE SHAPE OF MOLECULES ON THE BASIS OF VESPR THEORY
(i) Molecules with two bond pairs
In a molecule having two bonds pairs of electrons around its central atom, the bond pairs are located in the opposite side (at an angle of 180º) of the central atom so that the repulsion between them is minimum.
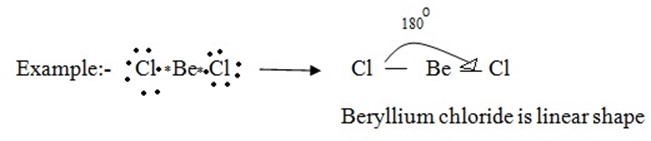
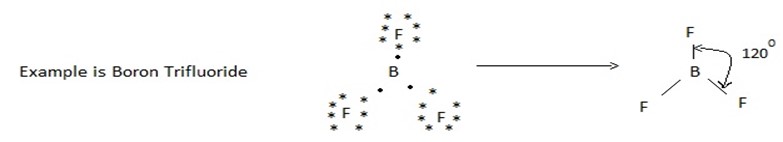
(ii) Molecules with three bond pair
In a molecule having three bond pairs of electrons around its central atom, the electron pair form an equilateral triangular arrangement around central atom. Thus the three bond pairs at 120º with respect of each other. Thus the molecule having three bond pairs around its central atom have trigonal planar (triangular planar shape).
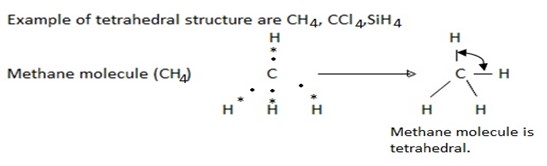
(iii) Molecules with four bond pairs
In a molecule having four bond pairs of electrons the four bond pairs are arranged tetrahedral around the central atom. Thus the four band pairs are at an angle pf 129º 28′ with respect to each other. Therefore the molecule having four bond pairs around its central atom has a tetrahedral shape.
(iv) Molecules with five bond pairs
Five bond pair orient themselves around the central atom in a trigonal bipyramidal way. Three bond pairs are in a plane called equatorial plane, and oriented at an angle of 120º with respect to each other.
Thus a molecule having five bond pairs around its central atom has a triangular bipyramidal shape.
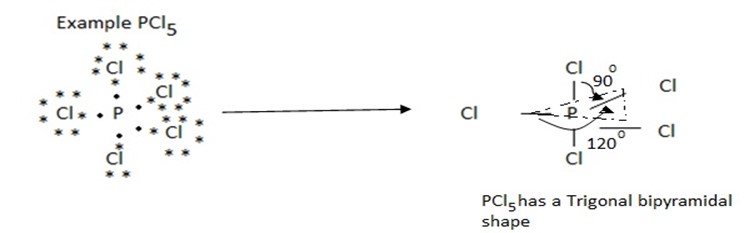
Therefore the molecule of the type shape AB5 are trigonal bipyramidal in shape e.g PCl5, PF5, SbCl5.
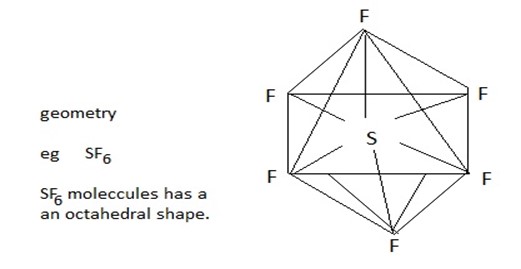
(v) Molecules with six bond pairs
Six bond pairs in a molecule are distributed octahedral around the central atom. Thus a molecule having six bond pairs around its central atom has an octahedral shape. Thus the molecules of the type AB6 are octahedral. Thus molecules SF6 has an octahedral
SHAPES OF THE MOLECULES HAVING BOND PAIRS AND LONE PAIR ELECTRONS.
The pair of electrons in the valence shell of an atom which is not involved in bonding is called lone pair of electrons.
For example the nitrogen atom in ammonia molecules (NH3) has one lone pair of electrons, the oxygen atom in water molecule (H2O) has two lone pairs of electrons.
(i) Molecules having three bond pairs and one lone pair.
A molecule having three bond pairs and one lone pair of electrons, thus has in all four pairs of electrons around its central atom.
Therefore these four pairs of electrons are distributed tetrahedral around the central atom.
(ii) A molecule of AB3 type has a triangular pyramidal shape
Typical molecules of this type are NH3, NF3,PCl3 and H3O+.
(iii) Molecules with two bond pairs and two lone pairs
The four electron pairs (two bond pair + two lone pair ) are distributed tetrahedral around the central atom
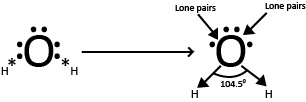
The two lone pairs are the central atom repel the bond pairs slightly inward due to greater lone pair-bond pair repulsion. As a result the bond angle in such a molecule is less than the tetrahedral vale of 109º 28′
The presence of only two bond pairs is the molecules gives a bent shape (inverted V- shaped) to the molecule. Example include H2O, H2S, F2O and SCl2.
(iv) Molecules with four band pair and two lone pairs
The four bond pair are distributed in a square planar nglish-swahili/distribution” target=”_blank”>distribution. The two lone pairs are in a direction at right angles to this plane.
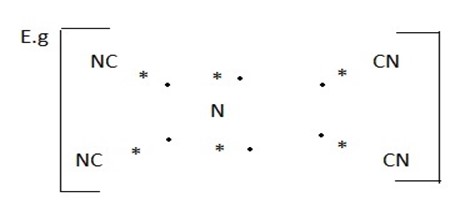
EXERCISE:-
(i) predict the shapes of the following molecules following the VSEPR theory.
(a)Ammonia (NH3) molecules
(b)beryllium chloride(BeCl3)
(c)water (H2O) molecule
(d)silicon tetrachloride (SiCl4)
HYBRIDIZATION
Definition:- Is the process of mixing of the atomic orbitals to form new hybrid orbital.
Note:- All hybrid orbitals of a particular kind have equal energy, identical shape and are symmetrically oriented in space.
CHARACTERISTICS OF HYBRID ORBITALS
(i) The number of hybrid orbitals formed is equal to the number of the atomic orbitals participating in hybridization.
(ii) All hybrid orbitals are equivalent in shape and energy but different from the participating atomic orbitals.
(iii) A hybrid orbital which takes part in the bond formation must contain only one electron in it.
(iv) A hybrid orbital, like atomic orbital cannot have more than two electrons. The two electrons should have heir spins paired.
(v) Due to the electronic repulsions between the hybrid orbitals they tend to remain at the maximum distance from each other.
TYPES OF HYBRIDIZATION
The types of hybridization shown by an atom depends upon the requirement of the reaction.
(a) SP3(ess-pee three) hybridization (or tetrahedral hybridization)
(b) sp2(ess-pee two) hybridization (or trigonal hybridization)
(c) sp (ess-pee ) hybridization (or linear hybridization)
(d) s,p & d) hybridization
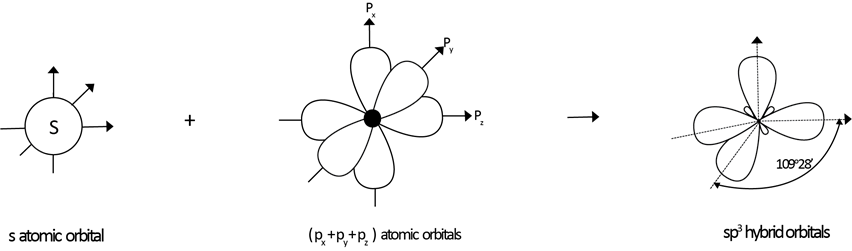
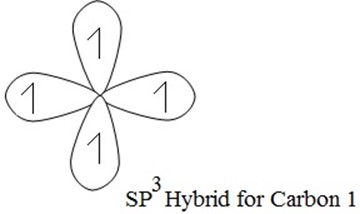
(a) SP3 HYBRIDIZATION
→ S + 3-P orbitals under mixing to provide new hybrid orbitals.
e.g S + (Px+ Py+ P2) → SP3
one s- orbital three p- orbital four hybrid orbital
Each of these hybrid orbitals has 25% S-character and 75% P-character. These four sp3 hybridized orbitals are directed along the four corner of a tetrahedron and are inclined to each other at an angle 109º 28′
Examples of sp3 hybridization is that of methane (CH4), the process of hybridization involves the following
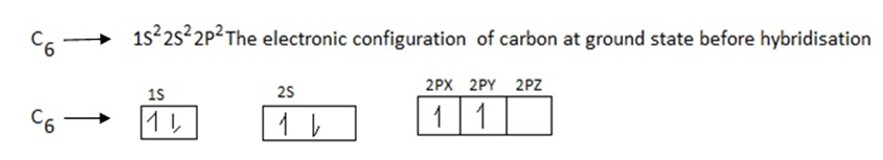
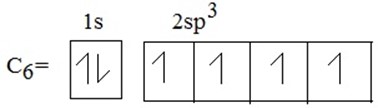
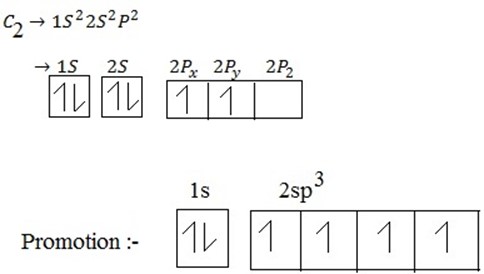
During exited state of carbon one electron is promoted from 2S to 2P orbitals .The process of promotion of an electron from 2s to 2p orbital of the carbon atom and subsequent hybridization is illustrated below.
During hybridization/ During excited state ,S is promoted to P orbitals as shown below:
Shape of S is spherical and shape of p is damp bell, then S hybridized with p shape.
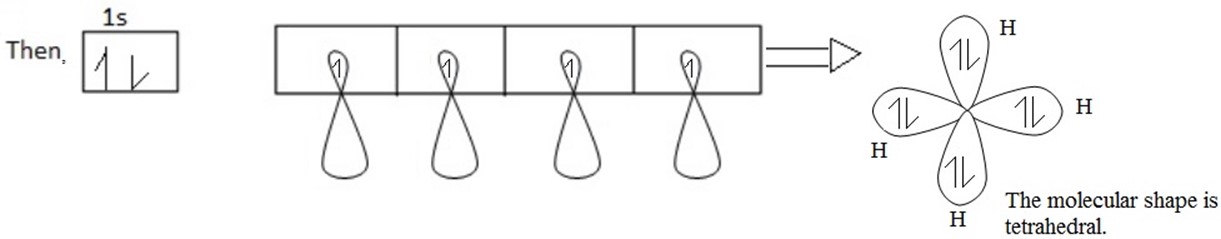
Hybrid between S + P
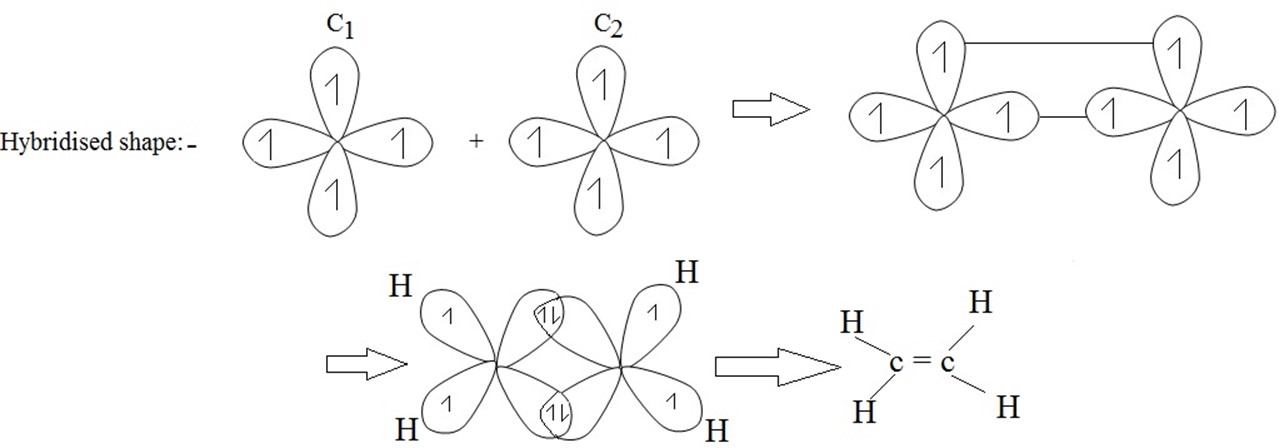
(b) SP2 HYBRIDIZATION (trigonal hybridization)
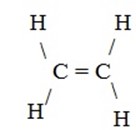
Example of SP2 hybrid orbital are the formation of ethane molecule(C2H4)
Note:- The three SP2 hybrid orbitals are oriented in a plane along the three corners of an equilateral triangle .i.e. they are inclined to each other at an angle of 120º the third p – orbital (say P2) remains unchanged each hybrid orbital has 33.3% s – character and 66.7% p – character
SP2 hybridization of Ethene (C2H4)/Ethylene
(i) Structure of ethane.
(ii) Electronic configuration of carbon.
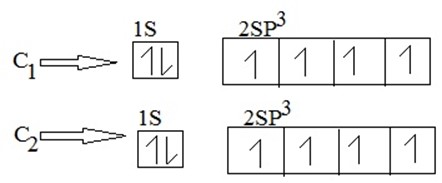
(iii) During excitation hybridization takes place. Then;
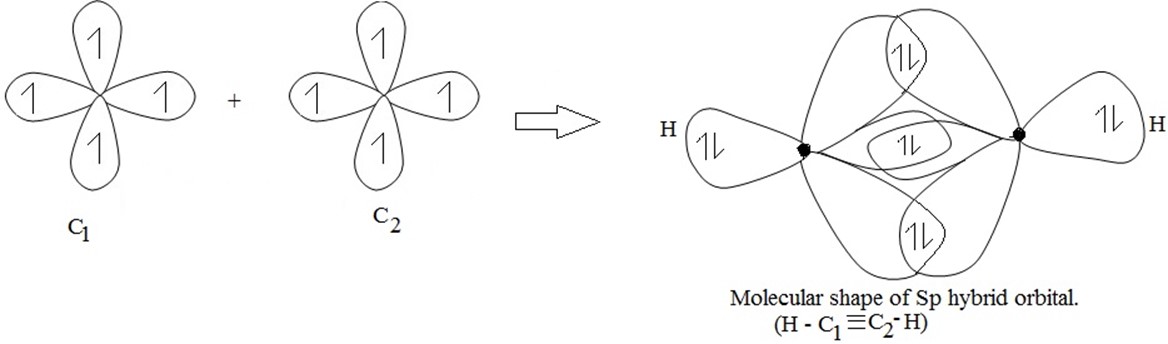
(c) SP hybridization(or linear hybridization)
Example of SP hybridization is the formation of ethyne(acetylene) molecules(c2h2)
(i) Structure of ethyne (acetylene)molecule
E.g (i) 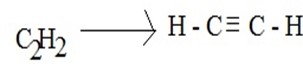
(ii) Electronic configuration of carbon from ground state.
(iii) During excited state , S is promoted to P – orbital to form hybrid orbital.
(a)
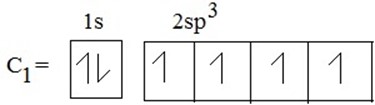
Hybrid shape for carbon 1
(b)
Hybrid shape for carbon 2
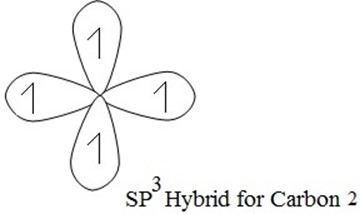
Then C1 combine with C2