HYDROGEN BONDING
If a hydrogen atom is bonded to a highly electronegative element such as fluorine , oxygen, nitrogen, then the shared pair of electrons lies more towards the electronegative element. This leads to a polarity in the bond in such a way that w slight positive charge get developed on H- atom.
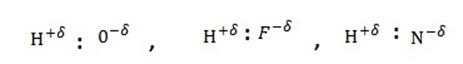
The bond between the hydrogen atom of one molecule and more electronegative atom of the same or another molecule is called hydrogen bond
CONDITION NECESSARY FOR THE FORMATION OF HYDROGEN BOND
(i) Only the molecule in which hydrogen atom is linked to an atom of highly electronegative element are capable of forming hydrogen bond.
(ii) The atom of highly electronegative element should be small.
SOME TYPICAL COMPOUND SHOWING HYDROGEN BONDING
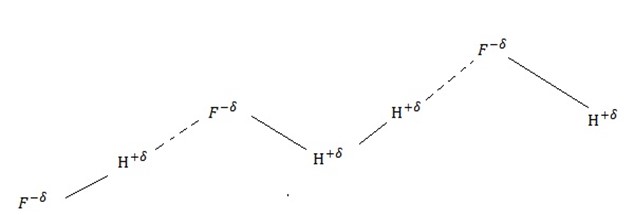
(i) Hydrogen fluoride( HF)
Structure of hydrogen fluoride:-
(ii) Water (H2O).
Water is a typical polar compound which exhibit hydrogen bonding as shown below:-
Structure of water molecule.
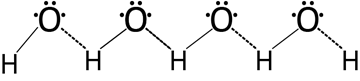
Hydrogen bonding that water exist in the associated form
TYPE S OF HYDROGEN BONDING
There are two types of hydrogen bonding
(i) Intermolecular hydrogen bonding
(ii) Intramolecular hydrogen bonding
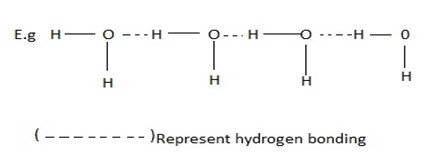
(i) INTERMOLECULAR HYDROGEN BONDING.
When the hydrogen bonding is between the h-atom of one molecule and an atom of the electronegative element of another molecule.
For example , hydrogen bonding in water, ammonia etc
(ii) THE INTRAMOLECULAR HYDROGEN BONDING
Is between the hydrogen of one functional group and the electronegative atom of the adjacent functional group in the same molecule. For example the molecule of o-nitrophenol show intramolecular hydrogen bonding. The p-nitrophenol shows intermolecular hydrogen bonding.
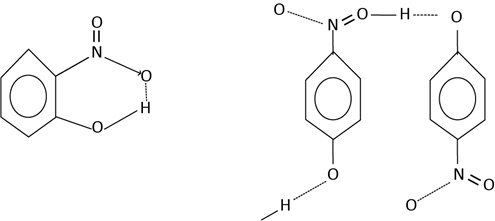
EFFECT OF HYDROGEN BONDING
Hydrogen bonding affects the physical properties of the compound appreciable .
Some effect of hydrogen bonding are described below:-
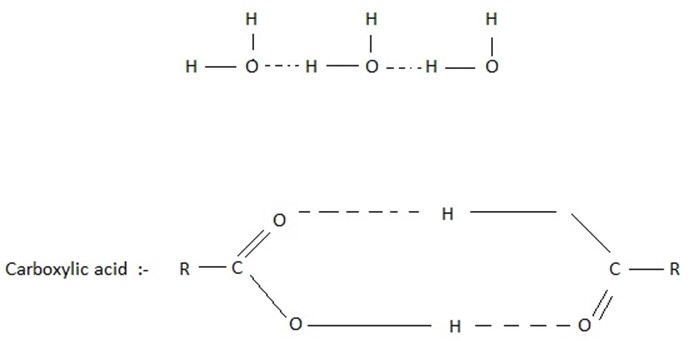
(i) Molecular association.
Formation of aggregates containing two or more molecules due to weak electrostatic interactions such as hydrogen bonding for example ,water molecule undergoes molecular association due to hydrogen bonding.
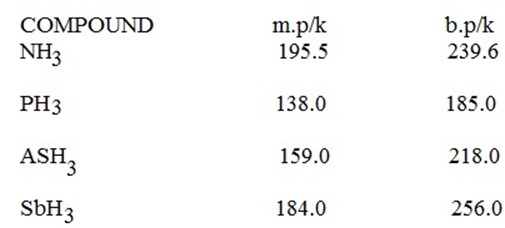
(ii) Increase in the melting and the boiling points.
The interaction which affected the melting and boiling points of NH3, H2O and HF is identified to be hydrogen bonding.
(iii) Influence on the physical state.
Hydrogen bonding affects the physical state of a substance also for example H2O is liquid while H2S is a gas under room temperature condition
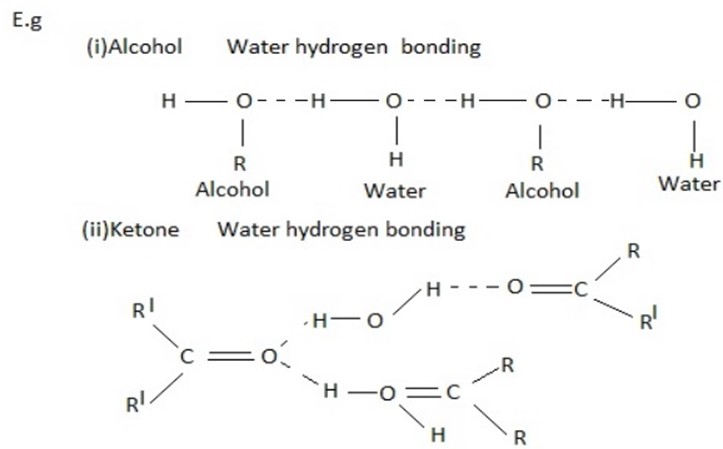
(iv) Solubility of covalent compound in water
Covalent compounds generally do not dissolve in water. However the covalent compound which can form hydrogen bonds with water readily dissolved in it. For example ethanol, Ammonia, Ammine lower aldehyde and Ketones dissolve in water due to their tendency to form hydrogen bond with water.
REVIEW QUESTIONS
Question 31
(a) Using the electronic configuration and periodic table, give the name of the element and the number of valence electrons.
(i) 1s2 2s2 2p4
(ii) 1s2 2s2 2p6 3s2 3p3
(b) Use Hund’s rule and other information to write out electronic configuration and orbital diagram of;-
(i) Cobalt
(ii) Nickel
(iii) Zinc
Solution
(a) (i) Oxygen: It has 6 valence electrons in the 2s and 2p sub levels.
(ii) Phosphorus: It has 5 valence electrons (in the 3s and 3p sub level)
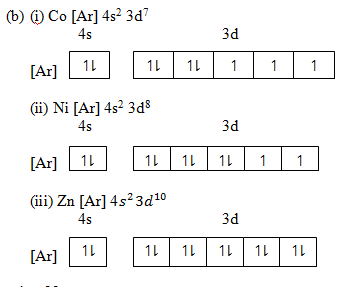
Question 32
(a) Provide the number of orbital in each of the following shells;-
(i) 1s
(ii) 2p
(iii) 3d
(iv) 4f
(b) Howe many sub – shells are there in each of the following sub – shells? K, N and L
(c) Explain if it is possible for an electron to have the following set of quantum numbers.
n = 3, â”” = 3; m; m = 3 and s = +1/2
(d) An electron is in a 4f orbital. What possible value for the quantum number can it have?
(e) What sub shell is found in the shell with n value = 4
(f) Write the possible value of the 4 quantum number for the outermost 2 electrons of Calcium.
(g) Write all the four quantum number for an electron added to Cl atom in forming Cl
(h) Write all the four quantum numbers for the outmost 2 electrons in Na
Question 33
(a) Write the total number of electrons possible for an atom whose n = 3. Assume that all the orbitals are full of electrons.
(b) Write electronic configuration of the following elements/ions
(i) O
(ii) K
(iii) Ni at number 28
(iv) Cu+ (at number of Cu = 29)
(v) Mo at number 42
(vi) Cl– (at number of Cl = 17
(c) What is the number of unpaired electrons present in the ground state of;-
(i) Fe+3 (At NO. of Fe = 26)
(ii) Cr3+ (At No. of Cr = 24)
(iii) Ni2+ (At No. of Ni = 28
(d) Why is the electronic configuration 1s2 2s2 2p2x 2p0y 2p0z not correct for the ground state of nitrogen?
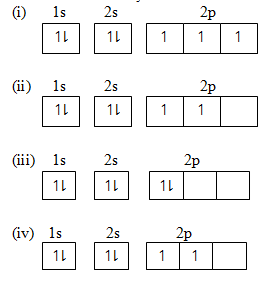
(e) Mention the disobeyed law from the following arrangements;-
Question 1
(a) State two major postulates and five short coming of Bohr’s atomic models.
(b) An electromagnetic radiation was emitted in the Balmer’s series as a result of electron.
Transitions between n= 2 and n= 5 calculate the;
(i) Energy of the radiation in kJmol-1
(ii) Frequency of the radiation in hertz
(iii) Wavelength of the radiation in metres.
(c) Calculate the wavelength of a line in the Balmer Series that is associated with energy transition
E4 E2 (E4 = -1.362 x 10-19J; E2 = -5.448 x 10-19J; h = 6.63 x 10-34J; C = 3 x 10-8 m/s)
Question 9
(a) The atomic spectrum in the visible is given by the following relationship: = RH
(i) What do symbols λ, RH, n1 and n2 represent? Show their SI units
(ii) Calculate the frequency of the third line of the visible spectrum i.e. a transit form n = 5 to n = 2 (RH = 1.097 X 107 M-1)
(b) By means of series of horizontal lines, draw an energy level diagram for visible atomic spectrum of hydrogen and indicate:
(i) The value of n for each line
(ii) By means of an arrow, show the transition which corresponds to the fourth line of emission spectrum in visible region.
(iii) By means of an arrow, indicate the transition which corresponds to the fourth ionization energy of hydrogen atom.
(c) (i) What do you understand by the term convergence limit and what is its significance?
(ii) Why are the discrete lines observed and not a continuous spectrum in Hydrogen spectrum?
Question 25
(a) Define the term electron configuration.
(b) Write down the electron configuration of these elements in their ground;
(i) Hydrogen 1p.
(ii) Beryllium.
(iii) Neon 10p.
(iv) Aluminium 13p.
(v) Calcium 20p.
(vi) Manganese 25p.
(vii) Cobalt 27p.
(viii) Zinc 30p.
(ix) Krypton 36p.
(x) Silicon 14p.
Question 18
Write electronic configuration diagram of the following;-
(a) S-2 (b) Na+ (c) Cr+3 (d) Cr+6 (e) Zn+2
Question 15
Write the electronic configuration of the following;
25V = [Ar] 4s2 3d3
16S = [Ar] 3s2 p4
9F = [He] 2s2 p5
34Cs = [Ar] 4s2 d10 4p4
16In = [Kr] 5s2 4d8
26Fe = [Ar] 4s2 d6
56Ba = [Xe] 6s2
Question 16
Provide electronic configuration of the following;
75Pt
80Br
68I
48Ga
92Cf
58Te
102Ra
17Cl
15P
30Zn
Question10
(a) All radiations are associated with the wave nature and differ from one another terms of their wavelength. Frequency, velocity and energy. Give the relationship between;
(i) Frequency and wavelength.
(iii) Energy and wavelength.
(b) Ozone (O) protects the earth’s inhabitants from the harmful effects whose wavelength is 2950A. Calculate
(i) Frequency.
(ii) Energy.
for this UV light
(c) If the wavelength of the first line of the Balmer series in a hydrogen spectrum is 6563, calculate the wavelength of the first line of Lyman series in the same spectrum. The uncertainty of the momentum of particle is 3.3 x 10-16gms-1. Find the accuracy in which its position can be determined.
Question 11
a) (i) A small object of mass 10g is thrown with velocity of 200 m/s given h = 6.626 x 10-34J.S. Calculate its wavelength.
(ii) Kinetic energy of a sub – atomic particles is 5.65 x 10-23J. Calculate the frequency of the particle wave (h = 6.626 x 10-34)
(iii) Calculate the momentum of a particle which has a de-Broglie wavelength of 0.1nm (h = 6.6 x 10-34 JS).
(iv) Two particles A and B are in motion, if wavelength λ of A is 5 x 10-8m. Calculate the wavelength λ of B. if its momentum is half that of A.
b) State and describe briefly Heisenberg uncertainty principle.
c) (i) Describe the concept that as the particle size gets smaller determination of its momentum and position simultaneous becomes difficult
(ii) Use the mathematical expression of the uncertainty principle to prove the validity of part c (i) above
d) (i) Calculate the uncertainty in position of an electron if uncertainty in velocity is 5.7 x 105 m/s
(ii) The uncertainty in position and velocity of a particle are 1010m and 5.27 x 1024ms-1 respectively. Calculate the mass of the particle.
Question 12
(a) Outline the postulates and weaknesses of Bohr’s atomic model.
(b) Calculate the mass of a photon of sodium light having wavelength of 5894A and velocity 3 x 108m/s given h = 6.6 x 10-34kgm2s-1 and 3.7 x 10-36kg.
(c) What’s the wavelength of a particle mass 1 gram moving with a velocity of 200ms-1 given h = 6.626 x 10-34J. (Answer 3.31 x 10-29m)
(d) The mass of an oxygen molecule is 5.3 x 10-26kg. Calculate its de Broglie wavelength if it is moving at a speed of 500m/s (Planks constant (h) = 6.626 x 10-34Js (Answer 2.5 x 10-11m)
Question 13
(a) Briefly explain the meaning of these terms, with the help of a diagram
(i) Wavelength
(ii) Frequency
(iii) Velocity
(iv) Amplitude
(v) Wave number
(b) Yellow light from sodium lamp has a wavelength of 5800Ao. Calculate the frequency and wave number of the radiations. Determine the range of frequency of visible light. The wavelength of radiations lying in the visible regions are between (3800 – 7600A0).
Question 14
(a) (i) What do you understand by dual nature of matter?
(ii) How does de Broglie equation consider the dual nature of matter?
(b) (i) Calculate and compare the energies of two radiations, one with wavelength 800nm and the other with 400nm
(ii) What is the amount of radiant energy associated with atomic 6.662 x 10-34Js, velocity of light 3 x 108m/s
(c) If the wavelength of a beam of light is 2.8 x 10-7m. Calculate it’s;-
(i) Wavelength in cm.
(ii) Frequency.
(iii) Energy of one of its photons.
(d) (i) Referring to Bohr’s atomic model, what’s ionization energy?
(ii) Calculate ionization energy of hydrogen. Reydberg’s constant RH = 1.097 x 107m-1
(e) (i) Calculate the frequency of the 3rd line of visible spectrum.
(RH=1.097 x 107m, C=3 x 108m/s)
(ii) Given that the wavelength of the 1st line of Balmer series is 6563A. Calculate the wavelength of the 2nd line in the spectrum.