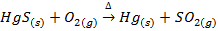Definition: Extraction of metals refers to the process of removing or obtaining metallic elements from their respective ores
What is an ores?
An ores is the mineral from which particular metal can be extracted conveniently and economically. example Haematite (Fe2O3), Iron pyrite (FeS2), galena (PbS), Zinc blende (ZnS), tin- stone or cassiterite (SnO2)
What are minerals?
- Are naturally occurring metallic compounds found in earth’s crust which can be obtained by mining, example Magnetite (Fe3O4), Gypsum (CaSO2.2H2O), Bauxite ( Al2O3.2H2O), Malachite (CuCO3.Cu(OH)2), Limestone (CaCO3) etc.
edu.uptymez.com
NB; All minerals are not ores but all ores are minerals
NATURAL OCCURRENCE OF METAL ORES
Metals occur naturally in two states
I. NATIVE OR UNCOMBINED STATE
II. COMBINED STATE
A. NATIVE STATE
The element are said to occur in the native state when they are found in their elementary form (free metals)
Example; Copper (Cu), Platinum (Pt), Gold (Au), Silver (Ag) and mercury (Hg)
B. COMBINED STATE
The elements are said to occur in combined state when they are found in the form of the compounds . Generally the reactive metals occur as oxides, sulphides, sulphates, silicates, carbonates, chloride, nitrates etc .The element that occur in combined state include Fe, Cu, Al, Pb, Sn, Ca, Mg, Na, Mn, Cr, Co etc
QUESTION; what are main ores from which tin, copper and aluminum are extracted?
NATURAL OCCURRENCE OF METAL ORES OF TIN, COPPER, AND ALUMINIUM
a) ALUMINIUM
Aluminium is the third element in abundance after silicon and oxygen. It is the most abundant metal in the earth’s crust. Aluminium is a reactive metal and hence does not occur as a free metal.
The following are different forms in which aluminium occur in nature;
I. Corundum(Al2O3) -Free oxide
II. Bauxite (AL3O3.2H2O) -Hydrated oxide
III. Silicates (Al2Si2O7.2H2O), KAlSi3O8, KH2Al3(SiO4)3
Kaolin (China clay) Feldspar Mica
IV. Cryolite (Na3AlF6)
Aluminium ores have different colours. The colours are due to the presence of other metals which are impurities, example Ruby (red) containing Iron and Titanium, Sapphire (Blue) containing cobalt and Titanium
Bauxite is the most economic ore from which Aluminium is extracted. Deposits of bauxite are exploited in South–East Europe, India, Australia, Brazil, USA, and West Indies
b) TIN (Sn)
Tin does not occur as free element because it is moderately reactive metal .Tin occur in form of oxide (tin-stone SnO2) and sulphide (tin –pyrite CuS.FeS.SnS2). The important ore of tin is cassiterite (tin- stone) this one contain (0.5-10)% of the metal as SnO2 and the rest are impurities i.e sulphides of Fe, Cu and lungstates of sand, Silicious matter, earth matter etc.
The miners call tin-stone as black tin to distinguish it from the white tin which has the name given to metal. Tin deposit occur in Malaysia, Indonesia, Bolivia, China, Burma, Thailand and Nigeria
c) COPPER (Cu)
Copper occur as free element as well as in combined state. In combined state copper occur as copper pyrite (CuFeS2), azurite (2CuCO3.Cu(OH)2) and malachite (CuCO3.Cu(OH)2). Malachite and azurite are basic carbonates. Deposit of copper occurs in Zambia, Democratic Republic of Congo. CuFeS2 is the chief ore of copper
NB; i) Copper occur as free metal in late superior in Canada
ii) Copper also occur naturally in form of oxide (ruby ore – Cu2O)
iii) Minerals and ores differ from one another due to the fact that minerals contain a low percentage of the metal while the ores contain a large percentage of the metal
iv) The metal cannot be extracted from the mineral; it can be extracted from an ore.
STAGES OF METAL EXTRACTION
There are three major stages of metal extraction
I. Concentration of ore
II. Reduction
III. Refining of purification
I. CONCENTRATION OF ORE
Concentration of ore means increasing the metallic content of the ore by removing the impurities (gangue). The ore which is mined usually contains large amount of rocky impurities such as sand, clay, limestone which are called gangue.
Definition; Gangue is the worthless or useless earthy impurities which are found in an ore.
Before concentration proper separation of ore is carried out, the ore is broken up into lumps which are then ground down to a fine powder which is suitable for the next operation. Concentration of the ore is carried out by any of the following methods depending upon the nature of the ore and gangue.
1) GRAVITY SEPARATION (WASHING WITH WATER)
This process is applied where there is well marked difference in the densities of the gangue and the ore. The crushed ore is washed by current water on a slopping table filled with a series of corrugated boards. The table is kept vibrating all the time. The lighter particles of gangue are washed off leaving behind heavier particles of the ore.
2) MAGNETIC SEPARATION
The magnetic impurities present in an ore are separated by a magnet .The powdered ore allowed to fall on a rubber belt moving around two rollers. One of the roller is a strong magnet, the ore moves with the belt towards the magnetic roller and then the belts take a turn near the magnet, the magnetic components is attracted by the magnet and form a separate heap. The non–magnetic components (e.g. tin-stone) is separated from magnetic component (e.g. Wolframite FeWO4).
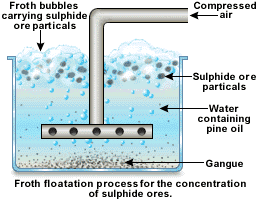
3) FROTH FLOTATION
This method is commonly used for concentration of the low sulphide ores like galena (PbS), copper iron pyrite (CuFeS2 or Cu2.Fe2S), Zinc blende (ZnS). This method of concentration is based on the different wetting characteristics of the sulphide ore and the gangue particles.
The finely powdered ore is mixed with water in a tank (floatation all). Some amount of pine oil (froth) and sodium ethyl xanthates collectors are added to it. Air is then blown through the mixture vigorous. The sulphide ore particles which are wetted by the oil rise up to the surface of liquid. The sulphide ore from stable froth which is skimmed off. The gangue particles which are wetted by water sink to the bottom of the tank (floatation cell). The froth with sulphide particles are collected in a separate tank
NB; The role of sodium ethyl xanthate is to collect sulphide ore particles. This compound is attached to sulphide ore particles and makes them water repellant. As a result the sulphide ore particles pass on into froth easily.
4) LEACHING
In this method the ore containing impurities is leached with aqueous solution of a suitable dissolving reagent so that the metal in an ore is converted into simple salt or complex compound while the impurities remain insoluble in the reagent which ore removed by filtration. The metal is then extracted from the simple salt or complex compound formed example impurities in bauxite (Al2O3.2H2O) i.e FeO, Fe2O3 and SiO2can be removed by heating. The crushed bauxite with sodium hydroxide solution (Bayer’s process). Aluminium present in the ore is converted into a soluble complex compound (aluminate complex –NaAlO2) while the impurities (i.e FeO, Fe2O3 and SiO2) remain insoluble and hence are filtered out.
5)
CALCINATION OF THE ORE
Calcination of ore refers to the process of heating the concentrated ore strongly in a limited supply of air or in the absence of air. The process of
Calcination brings about the following changes;
a) The carbonates ores are decomposed to their representative metal oxide
e.g.
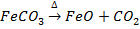
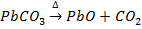
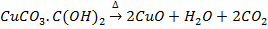
b) Water of crystallization in the hydrated oxide gets lost in form of water vapour.
eg.
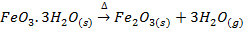
Limorite
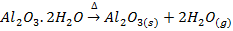
Bauxite
c) Organic matter, if present in the ore gets lost or expelled and the ore becomes porous
6) ROASTING
Roasting is the process of heating the concentrated ore strongly in excess air or oxygen below its melting point. Roasting can be done at moderate or high temperature. The roasting is generally for sulphide ores, when roasting takes place at moderate temperature some portion of the sulphide ore like galena (PbS), Zinc blende (ZnS) is converted into metallic oxide and remaining portion is converted into metallic sulphates.
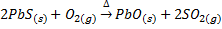
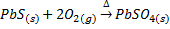
Also 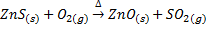
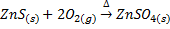
When roasting takes place at high temperature in the presence of oxygen, some sulphide ore give metallic oxide.
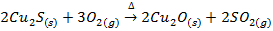
The sulphide ores of some metals like Cu, Pb, Hg, Sb etc when heated strongly in excess of air or oxygen are reduced directly to the metallic elements
E.g. 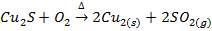
Copper glance
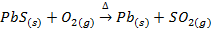
Galena