PROCESS OF COLOUR FORMATION
In isolated Transition atoms all five 3d – orbitals are degenerate and have equal energy. In presence of ligands the d- orbitals split into two degenerates i.e.
“Double degenerate and Treble degenerate” and which have different energy. Ligands exert electric field (Repulsion force) to unpaired 3d electrons.Repulsion cause orbital to split down into two degenerates. Double and Treble degenerates.
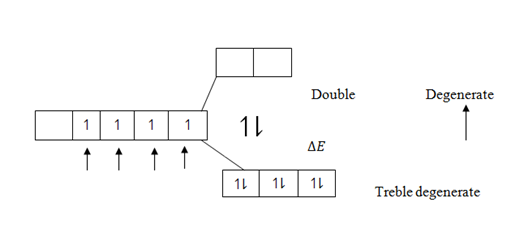
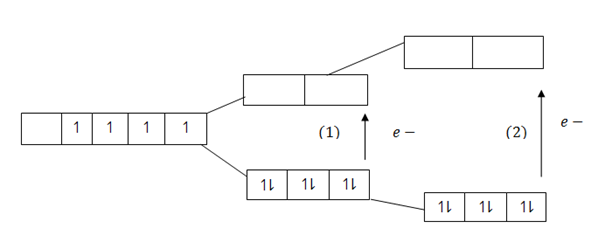
The energy of separation ( E) between the two degenerates is small. Such that the normal Radiant energy absorbed from the sun is enough to make electrons jump from treble degenerates orbital to double degenerates orbital and atoms excited, electrons fall back to their ground states, the process which is accompanied by emission of heat energy absorbed from the sun. The energy emitted by the falling electrons comes out in the form of radiation whose wave have specific definite colour.
E) between the two degenerates is small. Such that the normal Radiant energy absorbed from the sun is enough to make electrons jump from treble degenerates orbital to double degenerates orbital and atoms excited, electrons fall back to their ground states, the process which is accompanied by emission of heat energy absorbed from the sun. The energy emitted by the falling electrons comes out in the form of radiation whose wave have specific definite colour.
NOTE:
Intensive of the colour whether to be faint or deep it depends on electric field and splitting power of the ligand. If the ligands exert weak electric field (Weak Repulsive forces) the d – orbital will split and separates to a small extent, The energy of separation 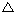 E becomes very small.
E becomes very small.
If the energy of separation  E between the two degenerates is small. Low energy will be required to excite electrons and make them jump to double degenerate orbital.
E between the two degenerates is small. Low energy will be required to excite electrons and make them jump to double degenerate orbital.
Similarly, low amount of heat energy will be emitted in the form of radiations when the excited electrons fall to their ground state.Intensity of the waves in the radiations will be low and hence the resulting colour appears to be light or faint. Ligands which exert weak electric field and cause small separation of the d orbital resulting to formation of light or faint colour are said to be “Ligands of high spin” .Ligands of high spin include Oxygen containing Halogens E.g. OH–, CL–, Br etc.
On the other hand if ligands exert strong electric field (strong repulsive force) the d – orbitals split down and will separate to a large extent. The energy of separation (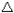 E) becomes large. In this case large heat energy will be required to excite electrons. Equally well large energy is emitted in the form of radiation, when the electrons fall back to their ground state. Intensity of the waves in the coming radiation will be high and hence the resulting colour appear deep.Ligands which exert strong electric field and cause large separation of the d- orbitals resulting to the formation of deep colours are said to be “ligands of low spin” Ligands of low spin include Nitrogen containing compound e.g NH3, CN–, CN etc.
E) becomes large. In this case large heat energy will be required to excite electrons. Equally well large energy is emitted in the form of radiation, when the electrons fall back to their ground state. Intensity of the waves in the coming radiation will be high and hence the resulting colour appear deep.Ligands which exert strong electric field and cause large separation of the d- orbitals resulting to the formation of deep colours are said to be “ligands of low spin” Ligands of low spin include Nitrogen containing compound e.g NH3, CN–, CN etc.
1.Ligands of high spin
·Extent weak electric field
·d- orbitals split to small extent
· 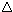 E energy of separation is small
E energy of separation is small
·The colour is faint or light.
2.Ligands of low spin
·Extent strong electric field
·D – orbitals separates to a large extent
· 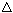 E is large (energy of separation)
E is large (energy of separation)
·The colour is deep.
NOTE:
It has been noted that colour formation by transition metals can be explained by two Theories.
(a)4s – 3d electron transition theory
(b)Crystal field theory.
Now which one is the TRUE THEORY between 4s – 3d electron transition theory and crystal field theory. The theory of crystal field is the True Theory and accounts better for colour formation by Transition metals
The following fact justify the statement
(i) Transition metals express their colours whenever they are in ionic form. In ionic form 4s – orbital is always empty. Since during ionization of the transition metallic atoms 4s electrons are given before 3d – electrons. This means that movement of electrons between 4s and 3d orbitals is not possible.
(ii) 4s – 3d electron transition theory cannot account for change in colour intensity. But crystal field theory accounts for change in intensity depending on the type of ligands weather are of high or low spin.
(iii) 4s – 3d electron transition theory cannot explain why SC 3+, Zn 2+ and Cu+ are colourless in aqueous solution but crystal field theory can account for this observation.
(iv)Observation has shown that Sc+ and Sc2+ are coloured in aqueous solution because the two ions have one unpaired electron in sub energy level 3d.Sc = (Ar) 4S2 3d1 has no electron in 3d orbitals. All the five orbitals in Sc3+ are empty. I.e. Sc3+ = [Ar] 4S0 – 3d0.
When ligands come with their lone pairs there will be no repulsion since d – orbital have no electrons.So no splitting of the d – orbital and no d – electron transition in Sc3+ Cu+ and Zn2+ are colourless because all the five 3d orbitals on these ions are full occupied by two electrons ie [sub energy level 3d in Cu+ and Zn2+ have Ten electrons]. No unpaired electron in 3d – orbital of Zn2+ and Cu+.
NOTE:
Despite the fact crystal field theory explains better colour formation by transition metals than 4s -3d electron transition theory yet it has some weakness. Crystal field theory fails to account for colour formation in Manganese (ii) ion (Mn2+). Manganese (iii) ion has unpaired electrons in its 3d – orbitals but in aqueous solution it is colourless
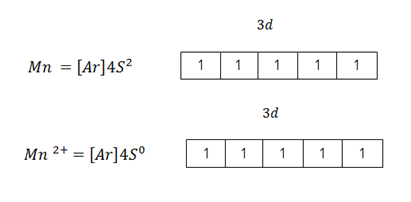
Manganese in the permanganate ion [MnO4–] has no unpaired electrons in the 3d – orbitals. Manganese in the permanganate ion has an oxidation state of +7. Oxidation state of +7 is attained after losing all the 4s and 3d electrons. However permanganate, ions in aqueous solution are coloured, make the aqueous solution PURPLE.
[B] COMPLEX COMPOUND
Is a compound which contain central atom and several ligand. The complex compound consists of central atom attached to the several atoms or group of atoms .
Central Atom
Is a metallic ion which accepts or accommodate pair of electrons during formation of complex compound. Most of central atoms are transition element.
The characteristics features which result into a transition metal or another metal to form a complex compound include the following:-
· They have vacant orbit or empty orbital which accommodate pair of electrons.
· They have high nuclear charge which exerts nuclear attractive force.
· They have small atomic size which exerts a strong nuclear attractive force.
These central atom or metallic ions have constant total number of ligand accommodate during complex compound formation. This is according to the number of vacant orbitals and atomic size of metallic ions. If the central atoms accommodate ligand above those required result the compound to be unstable. The following include the central
atom together with their total number of ligand.
Ag+ – 2
Hg+2 – 4
Al+3 – 4
Zn+3 – 4
Pb+2 – 4
Cu+2 – 4
Cr+3 – 6
Mn+2 – 5/6
Fe+2/+3 – 4/6
Ni+2 – 4/6
CO+2 – 4/5
Pb+2 – 4/6
LIGAND
Is a non metallic ion or molecules which donate pair of electrons to the central metal atom/ion during complex compound / ion formation. The non metallic ions or molecules have pair of electrons in the valency orbital. These pair of electrons called Ione pair.
There are two kind of ligand :
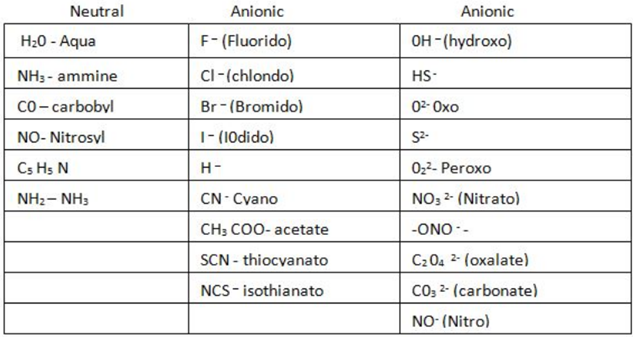
(i) Neutral ligand is a ligand which is electrically neutral. These are not charged ligand Such ligands are generally the molecular species having one or more lone-pair of electrons.
Eg.Amine NH3
Aqua H2O
Carbonyl CO
Ethane – 1, 2 – diaminie [en] NH2 – CH2 – NH2
(ii) Anionic ligands: Are ligand which are electrically charged.
These are ligands which carry negative charge on them.These charged ligand include the following:-
Fluoro F–
Chloro CI–
Iodo 1–
Cyano CN–
Hydroxo OH–
Nitro NO2–
Nitrate NO3
Carbonate CO3-2
Sulphate SO4-2
Oxalato C2O4-2
Anionic ligands generally form anionic complex ions. For example [Ag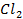 ]-, [Pb C
]-, [Pb C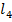 ]2-, [AL
]2-, [AL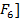 2- , [Ag(CN
2- , [Ag(CN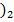 ]
]
Ligands can also be classified based on the mode of attachment.
(i)Monodentate (Unidentate) ligands: There are ligands which can attach to the central metal atom/ion only through one point.
(ii)Bidentate ligands:These are ligands which get attached to the metal ion through two points.
(iii)Tridentate ligands:These ligands get attached to the metal ion through 3 points.
(iv)Tetradentate ligands:
(v)Pentadentate ligands
FORMATION OF COMPLEX COMPOUND
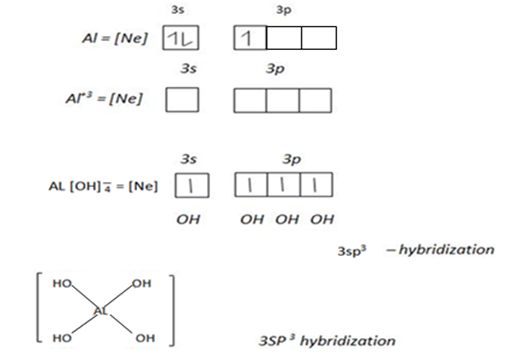
The central atom provide vacant orbit and ligand provide pair of electrons. In order for a central atom to form complex compound,first should lose electrons equivalent to their valency which become metallic ions then attract ligand. For transition element which form complex compound use the following vacant orbitals. For central atom which accommodate four ligand use ns and np vacant orbitals which result nsp3 – hybridization.
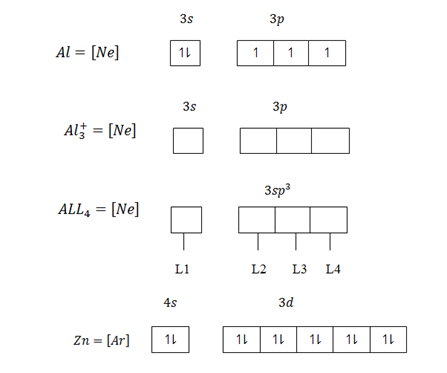
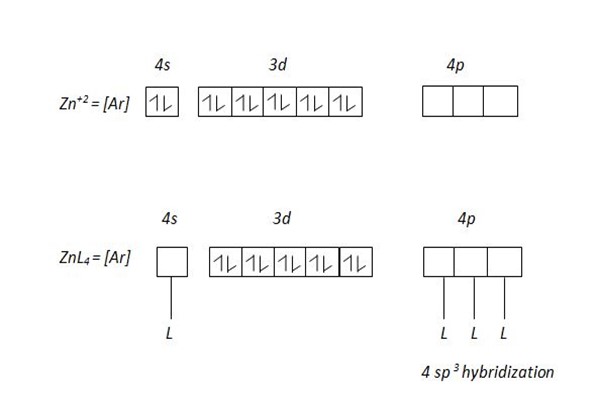
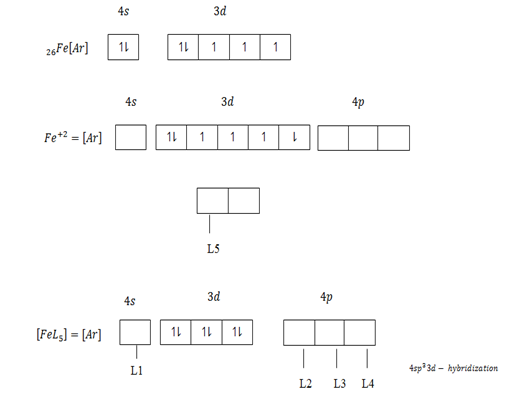
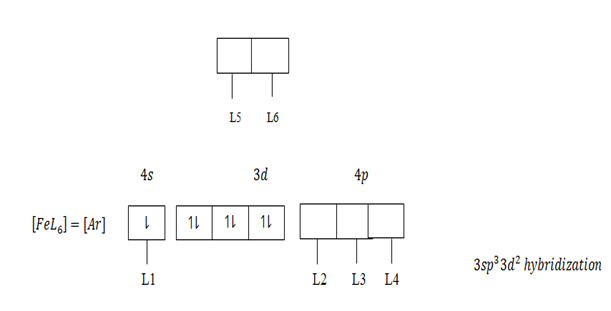
The central atom form complex compound if the ligand occurs in excess or high concentration Al+3 in a dilute or low amount of NaOH form simple compound Al+3 + NaOH → Al[OH]3 + Na+
But when the ligand occur in excess or high concentration cause Al3+ to form complex compound.
TYPES OF COMPLEXES
1. Cationic complexes are complex compound which are electrically positively charged. The total charge of central atom or metallic ions and all ligand result into the compound to be positively charged. Example of cationic complexes [ Cr Cl (H2O)3(NH3)2]+
2. Anionic complexes are complexes compound which are negatively charged. The total charge of metallic ions and all ligand result into the compound to be negatively charged. The total charge of metallic ions and all charge ligand result into the compound to have negative charge (Fe (CN)6]-6.
3. Neutral complexes are complex compound which are electrically neutral and has no net charge. The total number charge of metallic ions and all ligand form the neutral compound. [ Al(NH3) (OH)3].