RULES FOR NAMING INORGANIC COMPLEXES
The system adopted is that developed by the international union of pure and applied chemistry ( IUPAC).
i) Cations are always named before anions
The oxidation states of the central metal atom or ion is shown in Roman numerals in brackets immediately after it’s name.
Eg. [ Fe (H20) 6] Cl3 Hexaaquairon (III)Chloride.
ii) Within complex ,ligands are named first followed by the central metal ion . Ligands are named in alphabetical order.
Eg. [Co(NH3)4Cl2) Tetraamminedichlocobalt (III) ion
iii) The number of particularly are ligands present must be specified by using the following prefixes.
di – 2 ligands
tri – 3 ligands
tetra – 4 ligands etc.
iv) Names of negative ligands end up with suffix O. eg. CN– cyano ,Cl– chloro etc.
Neutral ligands usually retain their normal names except for the special cases : H20(aqua),NH3(ammine) etc
v) Anionic complexes end up in the suffix – ate often appended to the to the Latin or English name of the metal
Eg, [Co (CN)6]3- Hexacyanocobaltate (III) ion
[Fe (CN)6]3- Hexacyanoferrate (III) ion
vi) Cationic complexes use up their English name unchanged for the central metal ion.
Eg,[Cr(NH30)4cl2]+ Tetraamminedichlorochromium (III) ion.
Questions
Name the following complexes
(a) K4[Fe(CN)6]
Potassium hexacyanoFerric (ii)
(b) [Co(NH3)4(NO2]SO4
Tetra aminebromonitrocabalt (iii) sulphate
(c) (Cr(NH3)6(Cr(C2)4)3]
Hexaminechlomium (iii) trioxalatochromium (iii)
(d) [Al(H2O) (OH)5]-2
Aquopentahydroxoaluminium (iii)
(e) [Pt(en)2Cl2]-2
Dichlorodimetlydiamine platinum
(f) K[CoCl(H2O)NH3]5H2O
(1) + Cr + (-4) + (0) + 5(0) = 0
Cr = +3
(e) (Pt(en)2Cl2)
Dichrodimethlyamine platinum
(f) K [CrCL4(H2O) NH3] 5H2)
(1) + Cr + (-4) + (0) + (0) (5(0) = )Cr = +3.
Pentahydrate potassium amine aquatetrachlorochromate (iii)
Question
(a) Complex compound [CO(NH3) 5(Br)SO4
(i) Name the complex compound above
(ii) What the coordinate number of central atom
(iii) If all ligand placed by chloride ligand what is the charge of complex compound.
(iv) Write isomers of compound
(b) Using hybridization principles prove the following.
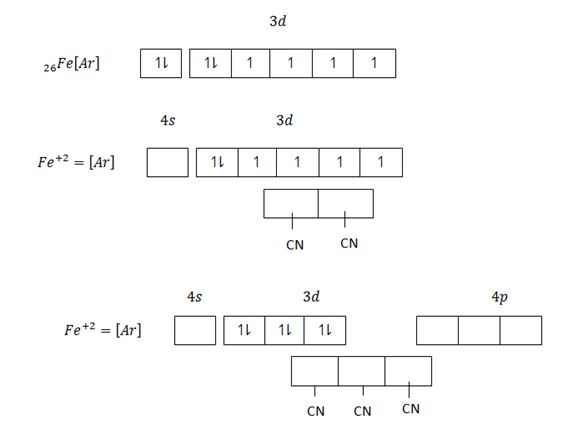
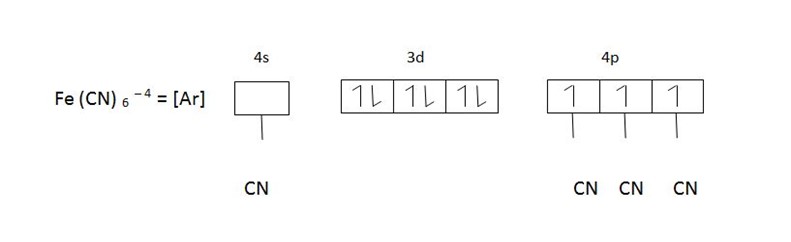
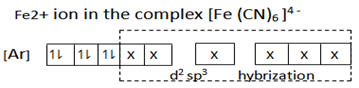
Fe(CN)6 -4 d2 sp3 hybridized
CO F6 -3 d2sp3 hybridized
Ni(CN)S-3 dsp3 hybridized
Solution
(a) [Co(NH3)5Br]SO4
(i) Diaminebromocobalt (iii) Sulphate
(ii) Co – ordinate number = 3
(v) [CO(NH3)Br]+2[COCl3] the complex compound so neutral
(vi) Isomers of complex compound
[CO(NH3)2Br]+2
[CONH3Br2]+1
Isomers of ligand obtain through changing the number of each ligand
(b) [Fe(CN)6]-4 d2 sp3 hybridization
Question:
Provide the IUPAC name of the following compound
(a) [Pt(NH3)3 Cl3] Cl
(b) Na (Al(CN)2]
(c) K6 (C02)(en)10 4H2O
(d) [Ni(H2O)4] [Ni(CN)4]
(e) [Mn(NH3)6] Cl3
(f) Na3(Cr) CN)6]Cl3
(g) Na2[Zn(CN)4]
(h) [Cr[H2O]5CL]Cl2.H2O
(i) CrCl2[H2O]4Cl.2H2O
Question
Consider the complex compound
K[Cr(NH3)2Cl2C2O4]
(a) What is the name of central atom
(b) List neutral ligand in the compound
(c) Name the charged ligand in the compound
(d) What is the name of complex compound
(e) What is the co – ordinate number of central atom
Solution
(a) Cr = Chromium
(b) NH3 = Amine
(c) Cl-1 = Chloro and C2O4-2
(d) Potassiumdiamminedichrolooxalatochromium (III)
(e) Co – ordinate number = 6
Question
Write the formula of the following complex compounds
(i) Dichlorotetra ammine cobalt (iii) chloride Tetraammine aquacopper
(ii) sulphate
(iii) Chloropentaaquacobalt (III) sulphate
(iv) Diaquatetraammine manganese (II) bromide
(v) Calcium di – iodo oxolatoferrate (III)
(vi)Trichlorotriammine chromium (III)
(vii) Trichlorotroammine planum (IV) chloride
(viii)Potassium disulphat atotetra aquachromate (III)
(ix)Tetrammine copper (II) sulphate monohydrate
(x) Potassium hepta axodichromate (vi)
(xi)Trichlorotriammine platinum (IV) chloride.
Solution
(i) [COCl2(NH3)2]+Cl–
P = [3] + [2] + [0] = 1
[COCl2 (NH3)2] Cl
(ii) [ Cu(NH3)4H2O]+2SO4-2
[2] + [0] + [0] = 2
[Cu(NH3)4H2O]+2SO4-2
Reaction of the following salt with dilute NaOH
| Salt solution | Small quantity | Excess |
| AgN.O3 Zn (NO3)2 Ca (NO3)2 Pb (NO3)2 Fe (NO3)2 KOH Mg (NO3)2 Al (NO3)3
|
Ag (OH) ppt Zn (OH)2 ppt Ca (OH)2 ppt Pb (OH)2 Fe (OH)2 – Mg (OH)2 Al (OH)3 |
Ag (OH) Soluble – Na2 (Zno)2) (COH)2 ppt Soluble – Na2 [pbo]2 Fe (OH)2 – Mg (OH)2 Soluble – Na[ACO2] |
edu.uptymez.com
NOTE: Precipitates of Zn, Pb and Al are able to dissolve in excess alkali because they form soluble complexes.
Reaction of the following salt with dil NH3 solution.
| Salt solution | Small quantity | Excess |
| Ag NO3
Zn (NO3)2 Ca (NO3)2 Pb (NO3)2 Fe (NO3)2 Cu (NO3)2 Mg (NO3)2 Al (NO3)2 |
Ag (OH) ppt + NH4 OH
Zn (OH)2 ppt + NH4 OH Ca (OH) ppt + NH4 OH Pb (OH)2 ppt + NH4 OH Fe (OH)2 ppt + NH4 OH Cu (OH)2 ppt + NH4 OH Mg (OH)2 ppt + NH4 OH |
[Ag (NH3)2] NO3CO
Zn (OH)2ppt Ca (OH)2ppt Pb (OH)2 – Insoluble ppt Fe (OH)2 [Cu (NH3)4] (NO3)2 Mg (OH)2ppt |
edu.uptymez.com
NOTE: All complex compounds are SOLUBLE
Bp is insoluble in excess NH3 (It form ppt)
Complete the following equations.
(i)Cu (OH)2(s) + NH3(ag)  [Cu (NH3)4] (NO3)2
[Cu (NH3)4] (NO3)2
(ii)Ag Cl (s) + NH3  No reaction
No reaction
(iii)Fe2 SO4 + KCN  [Fe (CN)6] SO4
[Fe (CN)6] SO4
GEOMETRIC SHAPE OF COMPLEX COMPOUND
The number of ligands direct coordinated to the metal ion is the coordination number,of that atom in the complex.
(a) Coordination Number 6:
Complexes belonging to this coordination number have octahedral structure’
Eg, [Fe (CN)6]4- hexacyanoferrate (II) ion
This is an inner orbital complex because the cyano – ligands are so strong that they push unpaired electrons inwards and force them to pair up.


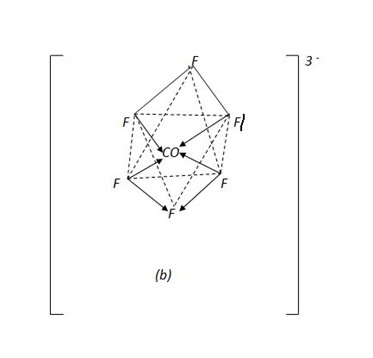
(b) [COF6]3- hexafluorocobaltate (iii) ion.
This is an outer orbital complex because the fluoro – ligands are weak. They cannot force electrons to pair inwards.
The six fluoro – ligands manage to occupy the orbitals in the 4th quantum shell.
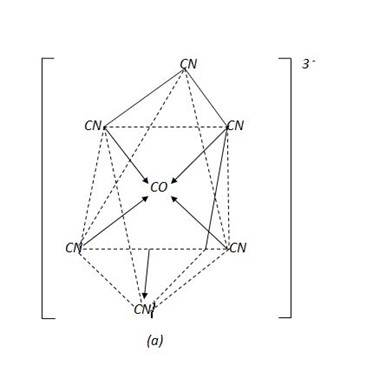
 (a) Octahedral structure of [Fe (CN) 6] 4-
(a) Octahedral structure of [Fe (CN) 6] 4-
(b) Octahedral structure of [COF 6] 3-
(b) Coordination number 5
Complexes with coordination number have trigonal bipyramidal structure .
eg. [Ni (CN) 5]3-
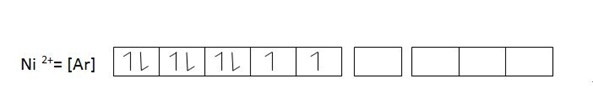
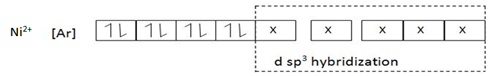
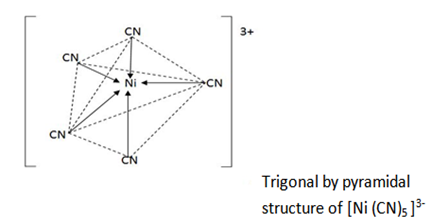
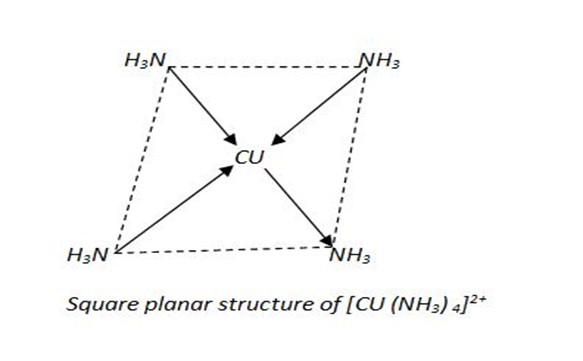
(C) Coordination number 4.
The most common complexes with this coordination number have square planar structure
eg. [Cu (NH3)4]2+
The shape 0f square planar complexes is flat
Question
Write the geometrical shape of the following complex.
(a) [Zn(OH)4]-2
(b) [CO(CN)3(OH)2]-2
(c) [Fe(H2O)2 (CN)4]–
(C)CATALYTIC ACTION
With exception to zinc, transition metals have catalytic action. They are used as catalysts in most chemical reactions catalytic power of catalyst is explained by their ability exist into stable oxidation states. In their catalytic ion, transition metals act as electron carries electrons in electron donors and pass them to electron acceptors.
Example iron and Nickel are catalyst during hydrogenation of alkene. Example V2O5, are used as catalyst during contact process. Example Manganese (iv)oxide used as catalyst during preparation of oxygen,iron used as a catalyst during Harber process.
(D) PARAMAGNETIC PROPERTY
Is a substance which can be induced with magnetism and can be attracted by magnetic bar. For a substance to be a paramagnetic it must possesses unpaired electrons in its electronic structure. Transitional metals with exception to zinc are paramagnetic. This is because they have unpaired electrons in 3d – orbital. Unpaired electrons form weak magnetic field and magnetic forces arise due to weak electric current so formed in the orbitals as a result of spinning of electrons in the orbitals.When a magnetic bar is brought near to a paramagnetic object there is a tendency of domains or unpaired electrons to shift from a side of weak magnetic field [from the object] to the side of strong magnetic field which is strong and the whole object appear to be attracted towards the [Magnetic field] magnet.
When electrons in the orbitals are paired there is interference and cancellation of magnetic fields occur as the magnetic field of two paired electrons move towards exactly opposite to each other (Vector) . All the magnetic fields cancel each other. Such substances cannot be induced with magnetic and cannot be attracted by a magnetic bar. Substances which cannot be induced with magnetic and cannot be attracted by a magnetic bar substances which cannot be attracted by an external magnetic field are said to be DIAMAGNETIC SUBSTANCES.
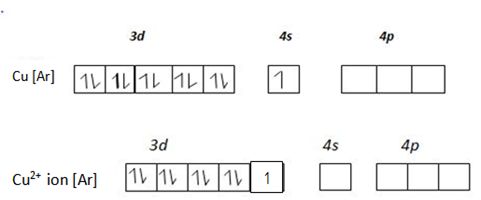
Transition metals are paramagnetic in pure metal or electrons are present in the delectronic structure of the transition metallic ion, for example Ferrous suphate [FeSO4] is paramagnetic just because Fe2+ has unpaired electrons in 3d orbitals.
This is because ligands of high spin when approaches the d – orbitals and cause small separation of the d – orbitals.The energy of separation ( E) between double degenerate and treble is small, electrons will spread in accordance to Hund’s Rule in which each electron occupy its orbital singly before pairing.
E) between double degenerate and treble is small, electrons will spread in accordance to Hund’s Rule in which each electron occupy its orbital singly before pairing.
In that case the central transition metallic ion remains with unpaired electrons in the electronic structure. Hence the complex combined will be paramagnetic. Consider Fe2+ ion when complexes with ligands of high spin like water [H2O]
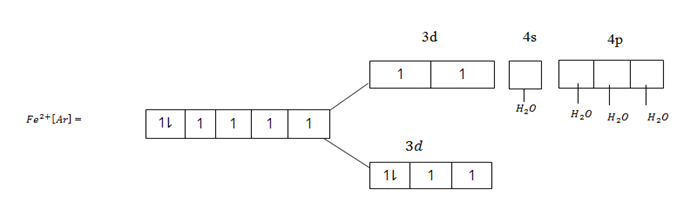
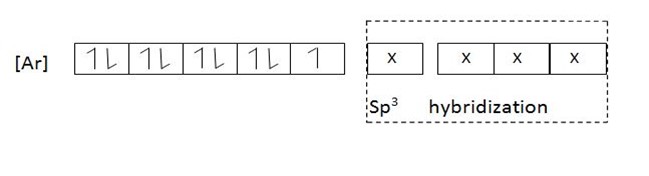
This complex [Fe(H2O4)4]2+ is a 4sp3 – hybrid complex and is paramagnetic. Since Fe2+ ion in the complex contains unpaired electrons in the d – electrons. If on the other hand Fe2+ complexes with ligands of low spin the paramagnetic property will be destroyed. The complex will be diamagnetic. Why?
This is because ligands of low spin split the d – orbitals and cause large separation.The energy of separation [  E] between the two degenerates becomes large.If the energy of separation is large electrons fill in the orbital in accordance to AUFBAU’S PRINCIPLE in which electron pair themselves in Treble degenerate orbitals, which have low energy according to AUFBAU’S PRINCIPLE. Consider Fe2+ complexes with ammonia which is ligand of low spin.This complex is 3d24sp3 hybrid complex and is diamagnetic since there are no unpaired electrons in electronic structure of Fe2+.All electrons Fe2+ are paired in Treble degenerate orbitals.
E] between the two degenerates becomes large.If the energy of separation is large electrons fill in the orbital in accordance to AUFBAU’S PRINCIPLE in which electron pair themselves in Treble degenerate orbitals, which have low energy according to AUFBAU’S PRINCIPLE. Consider Fe2+ complexes with ammonia which is ligand of low spin.This complex is 3d24sp3 hybrid complex and is diamagnetic since there are no unpaired electrons in electronic structure of Fe2+.All electrons Fe2+ are paired in Treble degenerate orbitals.
QUESTIONS
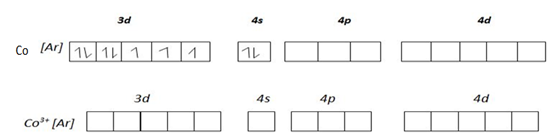
NECTA 2001 P2 Question 6c
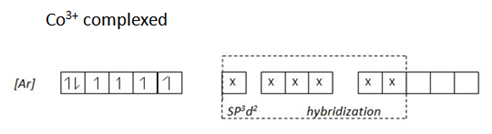
Use the configuration of 3d – orbital electron on cobalt (iii) ion to explain why [COf6]3- is paramagnetic while [COCN6] is not paramagnetic? [10%]
Solution
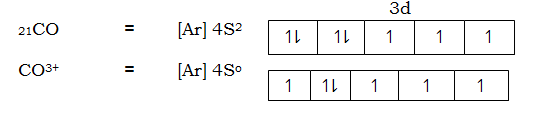
Consider electronic structure of CO and CO3+ ions given below
Then
Complex [CoF6] 3- is paramagnetic because fluoride ion is a ligand of high spin hence cause small magnetic field 3d – orbit of cobalt (iii) ion hence the energy separation is small and filling of electrons in according to Hund’s Rule, thus cobalt (iii) ion contains unpaired electrons in the 3d – orbitals. Hence a paramagnetic substance.
Consider
This is 4sp3 hybrid complex. And it is paramagnetic due to the in 3d – orbital
But
The complex [COCN6]3- is not paramagnetic due to absences of unpaired electrons in 3d – orbital in cobalt (iii) ion in the complex caused by complexing with ligands of low spin the cyano. This ligands exert strong magnetic field to the unpaired 3d – orbital hence large separation which cause excitation of be difficult hence filling of electrons is according to AUFBAU’S PRINCIPLE.
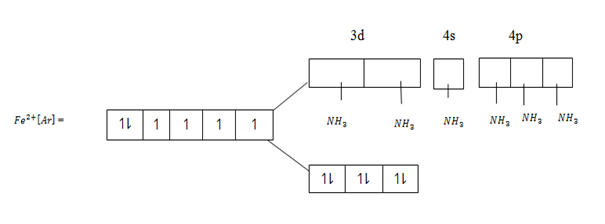
This complex is 3d24sp3 hybrid complex and is diamagnetic since there are no un paired electrons in electronic structure of Fe2+. All electrons Fe2+ are paired in treble degenerate orbitals.