QUESTIONS
NECTA 2001 P2 Question.6c
Use the configuration of 3d – orbital electron an cobalt (iii) ion to explain why [COf6]3- is paramagnetic while [COCN6] is not paramagnetic? [10%]
Solution
Consider electronic structure of CO and CO3+ ions given below.
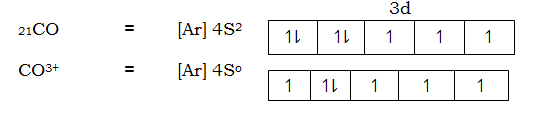
Then
Complex [CoF6] 3- is paramagnetic because fluoride ion is a ligand of high spin hence cause small magnetic field 3d – orbit of cobalt (iii) ion hence splits separates the hence the energy separation is small and filling of electrons in according to Hund’s Rule, thus cobalt (iii) ion contains unpaired electrons in the 3d – orbitals. Hence a paramagnetic substance.
Consider.
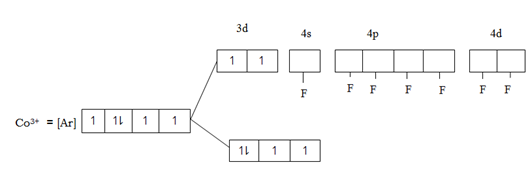
This is 4sp3 hybrid complex. And it is paramagnetic due to the in 3d – orbital
But
The complex [COCN6]3- is not paramagnetic due to absences of compared electrons in 3d – orbital in cobalt (iii) ion in the complex caused by complexing with ligands of low spin the cyano. This ligands exert strong magnetic field to the unpaired 3d – orbital hence large separation which cause excitation of be difficult hence filling of electrons is according to AUFBAU’S PRINCIPLE.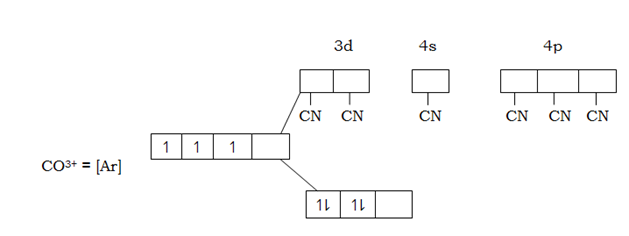
Paramagnetic property of transition metals can be destroyed in two ways
a. Temperature/ oxidation process
b. Complex compound formation
a. TEMPERATURE/ OXIDATION
Raise in temperature destroy magnetic property of the elements. Raise in temperature causes excitations of electrons and ionization of the atoms by losing electron.
Ionization to an extend of losing all unpaired electrons destroys magnetic property of the substance.
b. COMPLEX COMPOUND FORMATION
Formation of complex compounds by coordination with ligand may also destroy magnetic property of transition metals. However this will depends on oxidation state of the metals and nature of the ligands involved in company compound formation whether are ligand of high or low spin.
When the complex compound formed by ligand of high spin such as C2O4-2, OH-, F, Br, 1-, Cl- energy separation between treble and degenerate. Electron filled according to the Hund’s rule this result electron to be unpaired in the double and treble degenerate the complex remain paramagnetic example COF6-3 is paramagnetic substance. The F- filled in 4s, 4p and 4d vacant orbital the 3d remain with unpaired electron which result paramagnetic
Question
a. Predict the coordination number of Ni2+ and state whether the complex will be paramagnetic or diamagnetic it Ni2+ complex with
i) Bromine ions, Br – [Ligands of high]
ii) Ammonia molecules NH3 [ligands of low spin]
b. Fe3+ complexing with NH3 of low spin
C) VARIABILITY IN OXIDATION STATES
With exception to zinc, transition metals form more than one stable oxidation state. They have variable oxidation state. Variability in oxidation state in transition metals is explained by the ability to ionize by losing electron from both sub energy levels 4S and 3d.The energy present between 4s and 3d is very small. The gap existing between 4s and 3d is small. The different between the two degenerated is so small that just normal radiant energy from the sun is enough to excite electrons and so small that electrons from sub energy level 4s and 3d can be removed by almost the same amount of ionization energy. So that during ionization, transition
metals give off 4s – electrons first followed by 3d Transition metals depends on the number of electrons present in 4s – orbital. Chromium has lowest
oxidation state of +2, looses two electrons in 4s – orbital and copper the lowest oxidation state is +1. The maximum or the highest oxidation state is (achieved) attained after losing all the 3d electrons.
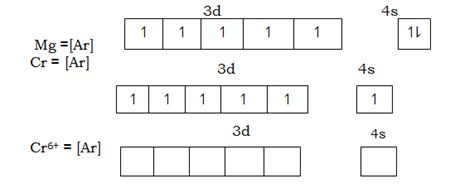
The extent of losing all the 3d – electrons is possible only for the first five elements from Sc to Mn. Elements beyond manganese can ionize to an extent of losing all the electrons because of large nuclear charge in these elements which cause strong effective charge pull, over the 3d electrons. The only oxidation state for elements found beyond manganese is +3 and for copper stable oxidation state is +2 which is attained after loss of the single 4s – electron and another from sub energy level 3d. The nuclear charge is high in zinc and hence the nuclear pull in 3d electron for zinc is maximum. Hence it is difficult to remove electrons from sub energy 3d in zinc. Zn has only one stable oxidation state of +2 which is formed after losing the two electrons. Only one stable oxidation state for zinc is one of the factors which excludes zinc from transition metals.
NOTE:
For these elements with atomic number 21 to 25 which can ionize to an extent of losing all the d – electrons, the tendency is that increase in oxidation states accompany with increase in acidic character. Lower oxidation states have basic character and higher oxidation states are acidic in nature. This is caused by increase in ionization energy needed to form the ions.The increase in acidic character with increasing oxidation states can be justified by considering the various oxides of chromium.
The various oxides of chromium include.
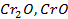
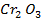
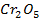 And
And 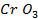
Base oxides amphoteric oxides Acidic oxides
The first two oxides, chromium (iii) oxide [Cr2O] and chromium (ii) oxide [CrO] are basic solutions which have no chemical reaction with other basic solution even alkaline solutions.

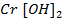
Then 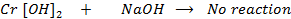
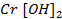 react with acidic solution
react with acidic solution
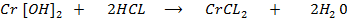
Chromium (ii) chloride
Chromium (iv) oxide [Cr2O3] is amphoteric. It dissolve in water and form hydroxides which dissolve in both alkaline solutions and acidic solutions.

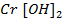
As base
Chromium (iv) hydroxide react acid solution
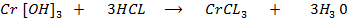
As acid
Chromium (iv) hydroxide dissolves in alkaline solution and form complex salt
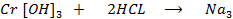 [Cr (0H) 6
[Cr (0H) 6
NaCrO3 + 3H20
Sodium chromide (iii)
The last two oxides, chromium (v) oxide Cr205 and chromium (vi) oxide (CrO3) are ACIDIC
OXIDES. They dissolve in water and form strong acidic solutions
CrO3 + H2O  H2CrO4
H2CrO4
Chromic acid
Chromic acid is so strong acid that has no chemical reaction with other acidic solution
H2CrO4 + H2SO4  reaction
reaction
Chromic acid reacts with basis solutions and form normal salts
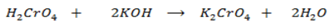
Reaction of the various oxides of chromium real justify with increase in Acidic character.
NOTE
Change in base acidic character in transition metals during ionization explain why Redox reactions take place either in acidic medium or basic medium, lower
oxidation states which are basic in nature, become more stable if the medium in which they are formed is acidic. On the other hand, high oxidation states which are acidic tend to be more stable if they are formed in basic medium. Therefore in redox reactions, when the elements change its oxidation states from high to lower oxidation state the process will take place in acidic medium from low oxidation state to high oxidation state, the process will occur in basic
medium.
SUMMARY
i. Low oxidation state  High oxidation state
High oxidation state
Basic in nature Mn2+ 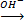 Mn04–
Mn04–
ii. High oxidation state 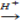 lower oxidation state
lower oxidation state
[Acid] MnO4-1
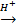 Mn2+ [base]
Mn2+ [base]
Purple Colourless
Or
Cr3+ 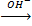 Cr2O72-
Cr2O72-
And
Cr2O7-2  Cr3+
Cr3+
–