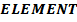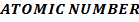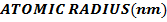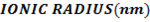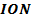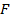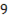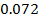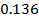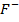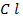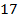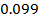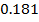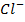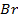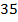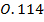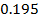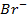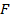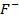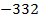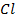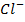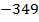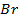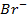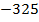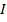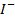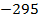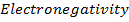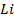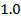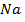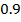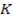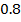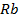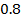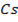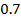II. Ionic radii
The ionic radius of the atom of an element is measured in the same way as the atomic radius. The ionic radii like atomic radii increases down the group. The reasons for this are exactly the same as those quoted for atomic radii. The radius of a cation is shorter than the radius of the parents atom (neutral atom) because electron or electrons have been removed and hence the force of attraction from the nucleus has increased e.g. Na=1.57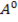 ,
, 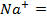 0.97
0.97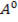
In contrast, all anions are larger than the corresponding neutral atom because electrons have been added to complete the noble gas structure. The added electrons reduces the force of attraction from the nucleus and as the result the size of an anion increase example Cl=0.99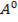 and
and 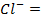 1.81
1.81 . The repulsive force of inner electrons also contribute to increase in ionic size.
. The repulsive force of inner electrons also contribute to increase in ionic size.
The table below shows the trend in ionic radii down group VIIA elements
NB: Ionic radius is half the distance between the nuclei of two ions in an ionic crystal
III. Ionization energy
This is the energy required to remove an electron from a gaseous atom or ion. Hence the first ionization energy of an element, M is the energy required to remove the first electron from it.
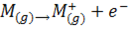
The second ionization energy is the energy required to remove the second electron from a gaseous ion.
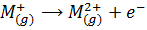
The successive ionization energies are defined accordingly .The higher this energy the tighter the electron is bound to the atom or ion. The ionization energy decreases down the group because OR due to the increase in atomic size of the element down the group, the increase in radii downs a group correlate with the decreasing ionization energies.
The nuclear charge increases down the group but it is cancelled by shielding effect and screening effect of the electrons of the inner shells. The two effects increases down the group. Since the nuclear charge is affected by the increase of electrons, the atoms become larger down the group and therefore the outer electron(s) is easily removed as it is loosely bound. Thus for the alkali metals Li to Cs, the outer most electron is most readily removed for Cs which is the largest element in group IA. Fluorine having the smallest atomic size in group VIIA has the highest ionization energy.
IV. Electron affinity (E.A)
The electrons affinity is the energy change which occur when an electron is added to a gaseous atom or ion. The non-metallic element readily gain an electron to give a negative ion.
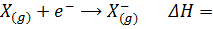
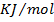
The energy associated with this process is termed as the electron affinity. The process may either absorb or evolve energy (example endothermic and exothermic respectively). Some elements evolve large quantities of energy i.e their electron affinities are large and negative. The more negative the electron affinity, the more the electron is attracted by the nucleus. Thus the first electrons affinity is the energy change which involve the addition of the first electron to a gaseous atom
 Negative electron affinity (in kJ
Negative electron affinity (in kJ )
)
The most negative electron affinity is found in Ground VIIA elements (I.e halogens) halide ions are most easily formed as they possess high electrons affinity. There is little variation in electron affinity down the group. Also there is no simple trend of electron affinity as shown in group VIIA. However there is general decrease in electron affinity down the group
NB: From the table above Chlorine has higher electron affinity than Fluorine. This is probability due to the relative small atomic size of Fluorine compared to that of Chlorine. The higher electron cloud in small Fluorine atom exerts a great repulsive force to the incoming electron giving rise to a smaller value of electron affinity. Chlorine with larger atomic size has smaller repulsive force and as result an electron can easily to be added to it and hence high electron affinity. All the electron affinity valves quoted for univalent ions is negative .To add an electrons to a univalent anion may require a large amount of energy in order to overcome electrostatic repulsion between the second electron and the charge on the anion. For example the second electron affinity of oxygen is positive.
The first electron is easily accepted

The2nd electron is not easily accepted

More energy required to shift with to higher energy level to create space for incoming electron. Thus energy released smaller than the energy gained
IV. Electronegativity
This is the measure of the attraction which an atom exerts on the electron pairs of a covalent bond. It can also be defined as the power of an atom in a molecule to attract electron to itself. The two atoms assumed to be involved in forming a single covalent bond where shared electrons are not equally attracted by the two atoms
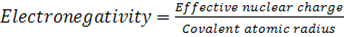
Electronegativity is directly proportional to effective nuclear charge and inversely proportional to atomic radius. Small atoms with relatively large effective nuclear charge tend to have large values of electronegativity. There is general decrease in electronegativity as one goes down a group. Therefore in the first element in each group (main group element) is much more electronegative than the rest. This account for much of the differences between its properties and that of other elements e .g Li is much more electronegative compared to other numbers of the group.
The following is the trend in electronegativity down group IA