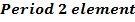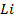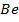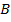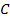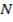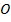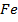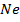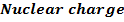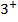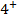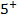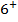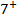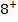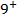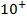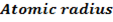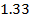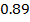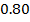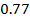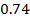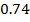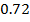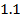2. ACROSS THE PERIOD
I. ATOMIC RADII(ATOMIC SIZE)
The atomic radii decrease steadily across each period. There is step rise in atomic size from halogens to alkali metals.
Consider period 2 elements
From the table above, it is observed that there is decrease in atomic radius from Li to F followed by an increase to Neon. This is probably due to the fact noble gases are mono atomic and their inter-nuclear distance is the distance between non bonded atoms held together by weak Van Der Waal’s forces. The decrease in atomic size gets smaller and smaller as shown in the table above. The same trends are observed in other periods
Generally atomic size (atomic radius) continue to decrease in passing along the period, Hence the alkali metal atom has the largest atomic radius than all the element in each period.
The halogen atom has the smallest atom radius of the elements in each period except iodine which has slightly larger radius than some of the transition metals in the middle of the period.
II. IONIZATION PERIOD
On moving horizontally along the periods we find that the first ionization energy increased. This due to the increase effective nuclear charge and decrease in atomic size for a small atom the outer most electrons are firmly held by the nucleus and hence much energy is required to remove them.
Across period 2 and 3, a contractions in radius correlates with the rise in ionization energy values. Abnormally high ionization potential values in Beryllium, Magnesium, Nitrogen, and Phosphorus are explained on the basis of the extra stability associated with full S and half filled P sub shell respectively.
A break is observed between Be and B in the period 2 and between N and O in the same period. In the period 3 we observe break between Magnesium and Aluminum and age between Phosphorus and Sulphur
These breaks can be accounted as follow;
a) There is a large increase in the ionization energy as we pass from it to He. This is due to the stability of the double state of Helium
b) The break in the trend of increasing ionization energy between Be and B is due to extraordinary large ionization energy of Be
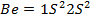
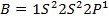
Be has two paired electrons in its 2S orbital. It needs energy first to split 2S electron pair and secondly to effect the electron removal. But for B there is only one electron in the 2P orbital which is easy to remove as it is more distant from the nucleus
A comparable explanation accounts for the break between N and O. Their electronic configurations are as follows;
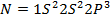
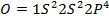
For Nitrogen there is extra stability due to half-filled P-sub shell. In Oxygen the 2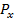 sub level has a pair of electrons which shields the
sub level has a pair of electrons which shields the 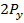 and
and 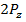 electrons rendering them easier to remove, hence an abnormally lower ionization energy for Oxygen. We may take almost similar account for the elements in period 3 where there is break between Mg and Al and also between P and S.
electrons rendering them easier to remove, hence an abnormally lower ionization energy for Oxygen. We may take almost similar account for the elements in period 3 where there is break between Mg and Al and also between P and S.
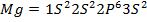
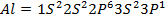
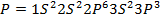
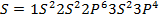
FACTORS AFFECTING IONIZATION ENERGY
1) Effective nuclear energy; The greater the effective nuclear charge the greater the ionization energy
2) Shielding Effect and Screening Effect; The greater the shielding and screening effect, the less the ionization energy
3) Radius ; The greater the distance between the nucleus and the outer electrons of an atom, the less the ionization energy
4) Sub level; An electron from a full or half –filled sub level requires additional energy to be removed
III. IONIC RADII
Ionic radii like atomic vary periodically with atomic number. The anions are much larger than the corresponding atoms while cations are usually much smaller. The explanation of the trends in atomic radii applies also to the very similar trends in ionic radii. Example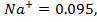
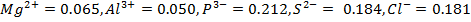
In period 4 the bivalent cation from Ti2+ to Z2+ show a d-blocks contraction similar to but larger than the corresponding contraction for atomic radius. The same is true of the Lanthanide contraction for the trivalent cations La3+ to Lu3+in period 6
vvvvvvv
IV. ELECTRONIC AFFINITY
Electron affinity along the period can be discussed in terms of non-metal and metals. It is generally observed that non-metal have higher values of electrons affinities than metals. Non-metals have higher values because they can readily gain an electron to give negatively charged ion. The process involving addition of electrons to neutral atom is exothermic because energy must be lost in order that the formed ion is stable.
Generally there is increase of electrons affinity across the group due to the increase of electronegativity which is a result of decrease in atomic radii of the respective elements. Also increase of effective nuclear charge increases electron affinity.
V. ELECTRONEGATIVITY
Electronegativity of the main group elements increase across each period. This is due to the increase in affective nuclear charge as well as decrease in atomic size. Therefore the halogens atom in every period has the highest electronegativity value than the rest members. Alkali are the least electronegative elements in each period.
VI. MELTING POINT
The melting point of an element is the measure of the amount of energy (heat) which must be supplied to breakdown the regular arrangement of atoms or molecule in crystal.
It`s a temperature at which a substance changes from solid into liquid. There are different types of force which holds atoms and molecules together, example due to such variation in forces the melting point of the elements in a period do not change uniformity e. g Period 3 elements. Melting point increase sharply from Na+ to Mg. Sodium atom to contribute only one electron to the metallic crystals, but Mg contributes two electrons. This accounts for the lower Melting point of Sodium compared to Magnesium.
All has three electrons in the outer valence shell but contributes only two of them to the “electrons sea”. This is why it has melting point close to that of Magnesium. The third electron is held firmly to the extent that it is not contribute to the “sea of electrons”
Silicon is non-metal with some metallic properties like luster, electric conductivity and ability to form alloy with metals. Silicon has the highest Melting point in period 3 due to its “giant covalent structure”. The silicon giant structure is comparable to that of Diamond.
Phosphorus and Sulphur have relatively low Melting point because their molecules are held together by weak Van Der Waal’s forces. Sulphur has a higher Melting point than Phosphorus due to the differences in sizes of their molecules i.e 
Chlorine is diatomic and is a gas at room temperature. Its melting point is very low due to a very low Van Der Waal forces holding the molecules.
.
PERIODIC TRENDS IN CHEMICAL PROPERTIES ACROSS PERIOD THREE (Na to Ar)
Chemical properties along the period depend on the change of the elements from strongly metallic to non-metallic. Sodium and Magnesium are strongly metallic while Aluminum is weakly metallic element. Silicon, Phosphorus, Sulphur and Chlorine are non–metallic elements. Thus metallic properties decrease across the period from left side of the periodic table to the right
a) HYDRIDES
The hydrides of period 3 elements include NaH, MgH2, AlH3, SiH4, PH3, H2S and HCl. Sodium hydride is strongly ionic while Magnesium hydride is largely ionic but the bonds are covalent. Aluminium hydride is covalent.
REACTION OF HYDRIDES WITH WATER
Sodium .Magnesium, and Aluminum yields hydrogen gas and metal hydrides, they are basic in nature because they react with water to form base
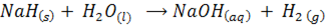
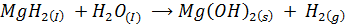
Silicon hydride (silane) evolve hydrogen with water in alkaline medium (catalyses the reaction)
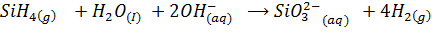
Phosphine (PH3) is a non–polar covalent compound and hence does not react with water. Phosphine is a non–polar covalent compound due to small difference in electronegativity values between Hydrogen and Phosphorus 
The hydrides of Sulphur (H2S) and Chlorine (HCl) are polar covalent compounds. They hydrolyze in water to form acid.
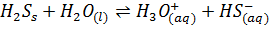
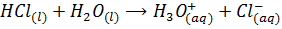
Therefore the hydrides of the strongly metallic elements tend to form alkaline solution with water while those of the non-metals form acidic solutions.