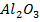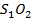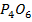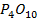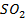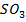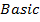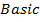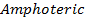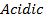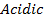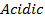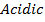b) CHLORIDES
The Chlorides of period 3 elements include NaCl, ,MgCl2, ,AlCl3, SiCl4, PCl3, PCl5, S2Cl2. The Sodium and Magnesium chlorides are ionic salts .The rest of the chlorides are covalent in nature. As metallic character decreases along the period ionic character of the chlorides decreases.
REACTION OF CHLORIDES WITH WATER
When the ionic chloride is added to water, there is an immediate attraction of polar water molecules for ions in the chlorides. The solid chloride either dissolves to form free ions example 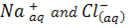 or react to form new substances.
or react to form new substances.
The hydrolysis of chlorides varies across the period Sodium chloride is not hydrolyzed in water probability due to a large size of Na+ ion leading to low polarizing power for water molecules. The chlorides of the rest elements have covalent characters. As metallic character decrease along the period, ionic character of the chlorides decreases as well.
The extent of hydrolysis of chlorides varies across the period. Magnesium chloride is not hydrolyzed but its hydrated crystals undergo hydrolysis when heated to give hydrogen chloride and a basic Magnesium chloride salt.
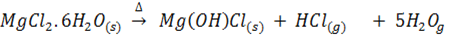
Aluminum chloride is easily hydrolyzed by water to produce an acidic solution. Al3+ ion is very small in size and it is highly charged. Thus its polarizing power is high, due to its high polarizing power it forms hydrated ions ([Al(H20)6]3+). The Al3+ ion strongly polarizes the O-H bond of the water molecules in the [Al(H2O)6]3+ ion to the extent that the bonds break to release the hydrogen protons. The solvent water molecules abstract protons from polarized water molecules to form the acidic hydroxonium ion H3O+, The H3O+ I is formed as follows
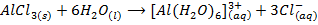
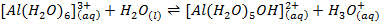
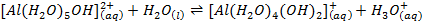
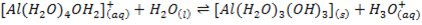
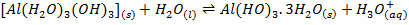
The chloride of Silicon Phosphorus and Sulphur hydrolyses completely in water to form acidic solution or solid
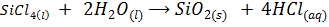
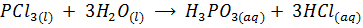
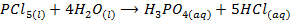
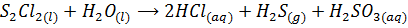
Note: As metallic character decreases along the period, ionic nature of the chloride decrease while the extent of hydrolysis increases
c) THE HYDROXIDES
The hydroxides in period 3 include NaOH, Mg(OH)2, Al(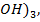 SiO(OH)2, P(OH
SiO(OH)2, P(OH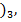 PO(OH )3,SO2(OH)2 and ClO3(OH),Cl(OH). The hydroxides from silicon to chlorine are not true hydroxide but they are oxy–acids.
PO(OH )3,SO2(OH)2 and ClO3(OH),Cl(OH). The hydroxides from silicon to chlorine are not true hydroxide but they are oxy–acids.
Sodium and Magnesium hydroxides are the true hydroxide. They have strong tendency of releasing the OH– group. Aluminum hydroxide is Amphoteric.
The acidic character of non-metal hydroxides is due to the tendency of releasing or donating H+ ion when dissolved in water. Silicon, Phosphorus, Sulphur and Chlorine are electronegative enough to withdraw electron by inductive effect from the O-H bond thus, facilitating the release of hydrogen as a proton H+.
The acidity of the hydroxide of Si, P, S and Cl increases with the increase in the electro negativity of respective elements. Solubility decrease from NaOH to 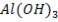 due to decrease in metallic character or ion character of the hydroxides across the period
due to decrease in metallic character or ion character of the hydroxides across the period
d) OXIDES
Sodium and Magnesium are strongly metallic and their oxides are ionic. Aluminum oxide is ionic but not basic as these sodium and magnesium.
The oxides of sodium and magnesium are strong bases while aluminum oxides are amphoteric. The oxides of the remaining elements are all acidic.
REACTION OF OXIDES WITH WATER
Solubility of the oxides of period 3 elements decreases along the period as metallic properties decreases. The oxides of sodium and magnesium form hydroxide with water or steam because of the protective film of oxide. The oxides of phosphorus sulphur and chlorine reacts with water to form acidic solution. Silica (SiO2) does not react with water but it’s acidic
E.g. 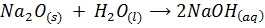
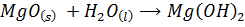
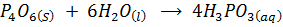
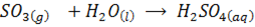
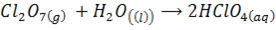
DIAGONAL RELATIONSHIP BETWEEN THE ELEMENTS
The first element in every group is the smallest and has the highest electronegativity compared to the rest group member as a result the first elements have properties which differ from the rest group member but similar to those at the next lower elements diagonally. The kind of relationship in which the elements which are diagonally located in the period’s table have similar properties is known as diagonal relationship.
Diagonal relationship may also be explained in terms of polarizing power of the diagonal elements .The polarizing power of an element is the ability of positive ion to polarize the negative ion .When positive and negative ions approach each other their shape are distorted. The extent by which the ion is able to undergo distortion is called polarizability. The effect is polarization is as follows;

NB; Polarization is the distortion or deformation of an electron cloud of an anion by a cation.
If polarization is quite small the ionic bond results and it is high the electrons in the anion are drawn towards the cation to the extent that a covalent bond is formed .The polarizability and polarizing power of an ion is affected by;
i. The size of the ion
ii. The charge on the ion
Thus polarizing power is high for a small ion which has high effective nuclear charge. On moving across a period from left to right, ionic radii decreases and effective nuclear charge increases. On descending a group ionic radii increases while the effective nuclear charge decreases. Hence the diagonal elements have similar polarizing power. Due to these elements have similar properties. The diagonal relationship can be represented as follows
Element Li Be B C N O F
Electronegativity 1.O 1.5 2.0 2.5 3.0 3.5 4.0
Element Na Mg Al Si P S Cl
Electronegativity 0.9 1.2 1.5 1.8 2.1 2.5 3.0
The relationship is most significant in the following pairs Li and Mg, Be and Al, Be and Si etc
LITHIUM AND MAGNESIUM.
Lithium resembles magnesium and differs from the other alkali metals as follows;
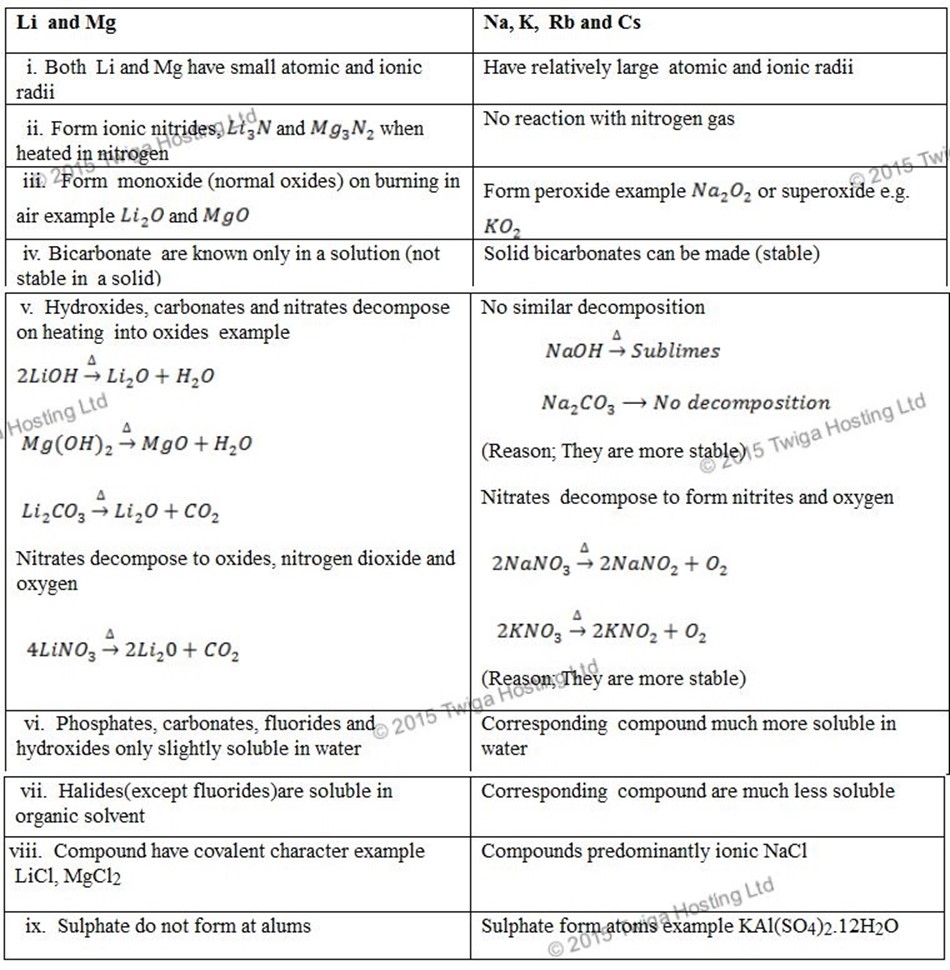
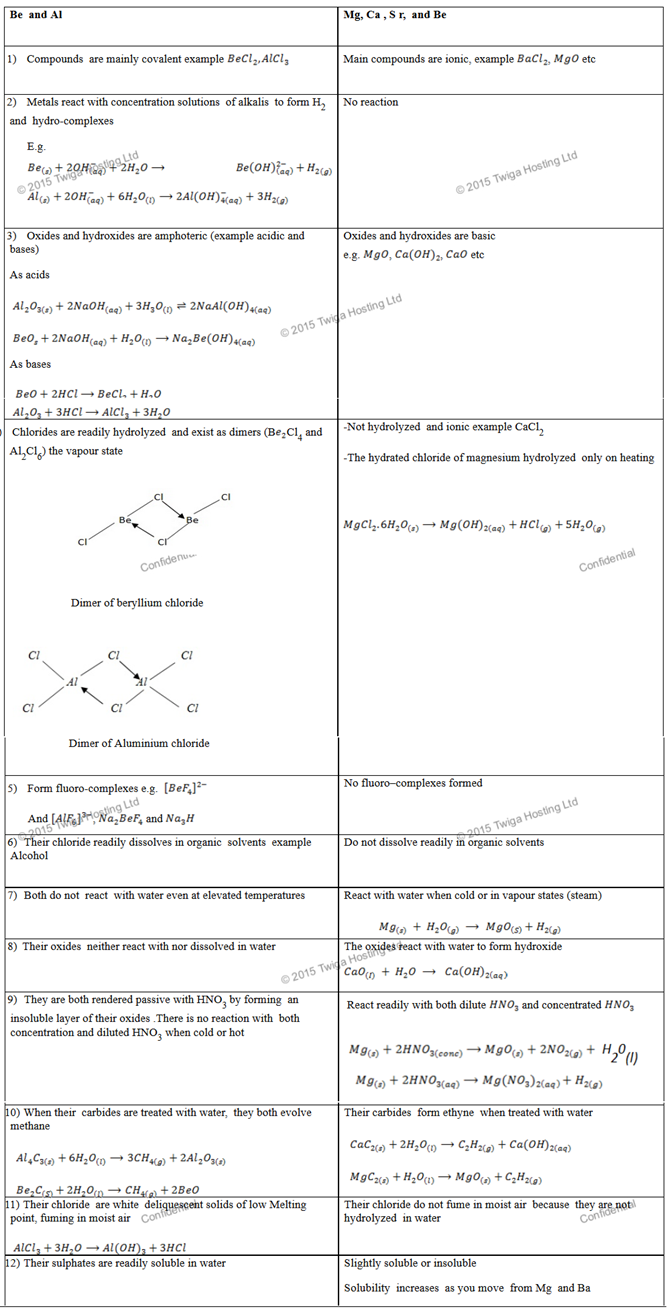
BERYLIUM AND ALUMINIUM