6. MIXED OXIDES
These are oxides composed of two simple oxides. The two simple oxides may be of the same metal or different metal in different oxidation states, example Red–Lead (Pb3O4) is combination of 2PbO and. Due to this can be written ionically
Also magnetite (Fe3O4) is a combination of FeO and Fe2O3
The mixed oxides with different metals are as follows;
Magnesium ferrite (MgFe2O4) MgOFe3+2O3
ZnFe2O4 nOFe23+O3 (Zinc ferrite)
PROPERTIES
- It is a brilliant scarlet (bright red) solid insoluble in water
- Red lead behaves chemically as if it were a loose compound of lead monoxide (PbO) and lead dioxide ( PbO2). For example it reacts with dilute HNO3 on warming to give and water whereby PbO2 is left with no reaction
edu.uptymez.com
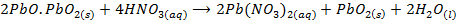
- The reaction above is a redox reaction indicating that is a reducing agent
edu.uptymez.com
USES; Red-lead is used as pigment in oil plants
FERROUS –FERRIO OXIDE (Fe3O4) (FeO.Fe2O3)
Fe3O4 occurs naturally as Magnetite. It may be prepared by heating iron with oxygen or steam
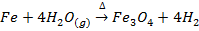
PROPERTIES
– The compound is black in color
– Very strongly ferromagnetic
– The compound is inactive chemically
– React with acids as a double oxide giving a mixture of ferrous and ferric salts in solution
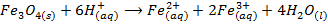
HYDROXIDES OF THE METALS
These are compounds of metals which contains hydroxide ions (OH–) as the only negatively charged ion, example NaOH, Mg(OH)2, Zn(OH, Fe(OH)3
PREPARATION OF METAL HYDROXIDES
There are two methods of preparation of metal hydroxides.
A) DIRECT METHOD OF PREPARATION OF METAL HYDROXIDES
The hydroxides which can be prepared by this method are those composed of strongly electropositive metals. Example LiOH, NaOH and Ca(OH)2
Example
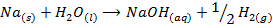
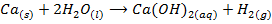
B) INDIRECT METHOD OF PREPARATION OF METAL HYDROXIDES
In this method the metal hydroxide is prepared by
a) The action of water on the metal oxide;
example
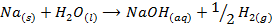
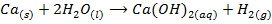
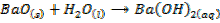
NB: the metal hydroxides which are prepared by action of water on metal oxides are soluble in water.
b) Action of calcium hydroxides (milk of lime) on a solution of carbonate, example preparation of NaOH and KOH. These metal hydroxides are prepared by precipitation the unwanted ions and layering a solution of the alkali. For instance when potassium carbonates and (Ca(OH) solution are mixed and then allowed to settle, a solution of potassium hydroxides may be decanted
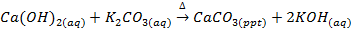
c) Precipitation of a metal hydroxide by adding ammonia solution or sodium hydroxide solution to a solution of salt of the metal
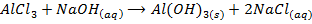
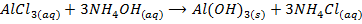
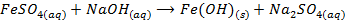
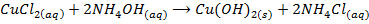
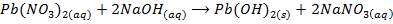
d) Electrolysis of a solution of the metal chloride, example preparation of NaOH. Alkali metal chlorides form conducting solutions and since these metals are highly in the electrochemical series, their ions remain in solutions during electrolysis and hydrogen evolved at the cathode. Preferential discharge of chlorides ions enable hydroxyl ions formed by ionization of the water to accumulates in the solution. As a result dilute solution of the metal hydroxide is produced
PROPERTIES OF METAL HYDROXIDES OF THE SELECTED METALS
1. ALKALI METAL HYDROXIDES (MOH)
PHYSICAL PROPERTIES
-The hydroxides of group IA metals are white crystalline solids
-They melt at moderate temperature without decomposition except (LiOH)
-They are deliquescent solids
-They are very soluble in water (form alkali solutions)
CHEMICALS PROPERTIES
-The basic strength of the alkali increases down the group, example calcium is the strongest base
-When cold and dilute alkali’s reacts with chlorine to form metal chloride and hydrochlorite
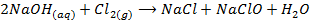
-When hot and concentrated alkali’s react with chlorine to form metal chloride and chlorate (v)
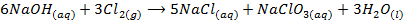
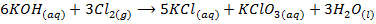
In the two reactions chlorine undergoes disproportion
– Most NaOH and KOH absorbs CO2 from the air whereby a metal carbonates is formed
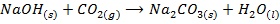
– The hydroxides of Group IA metal reacts with acids to form salts and water only, example undergo neutralization reaction
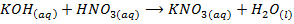
USES OF HYDROXIDES OF Na AND K
- Owning to their highly basic character alkali metal hydroxides are used to absorb acidic gases, example CO2
- Alkali metal hydroxides are used in neutralization reaction.
edu.uptymez.com
Example
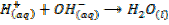
- Alkali metal hydroxides are used in precipitation reaction
edu.uptymez.com
Example
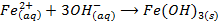
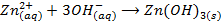
- Caustic soda (NaOH) is used in the manufacture of silk, paper and soap
- Caustic potash (KOH) is used to manufacture soft soaps
edu.uptymez.com
2. ALKALINE EARTH METAL HYDROXIDES (M(OH)2)
PHYSICAL PROPERTIES
– They are white crystalline solids
– Solubilities increases considerably down the group from beryllium hydroxides (Be) to barium hydroxides (Ba). Beryllium hydroxide is insoluble in water.
Solubility of calcium hydroxide decreases with rise in temperature, the others increase, magnesium slightly but strontium and barium hydroxide greatly. Increase in solubility down the group is due to the fact that lattice energy decreases faster than hydration energy (Be() is essentially covalent because of the high polarizing effect of the small
– Group IIA hydroxides are much less soluble
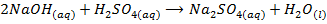
-The hydroxides of Na and K precipitates some metals from their soluble salts (example, Aqueous solutions of their salts) as hydroxides
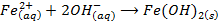
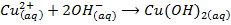
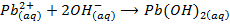
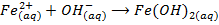
– Both NaOH and KOH liberates ammonia gas when added to ammonium salts
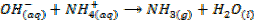
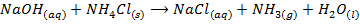
REACTION WITH AMPHOTERIC METALS
Zinc, Aluminium, Lead and Tin react with hydroxides of sodium and potassium to form complexes, example aluminate, plumbate, zincate and stumnate.
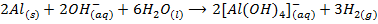
Aluminate ion
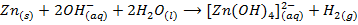
Zincate ion
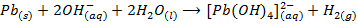
Plumbate ion
REACTION WITH CARBONDIOXIDE
When CO2 is bubbled through aqueous solutions of the NaOH and KOH the carbonates are formed,With excess of the CO2
the hydrogen carbonates are formed.
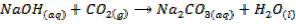
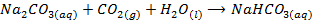
Of group IA elements due to the decrease in metallic character of the elements (example Group IA elements are more electropositive than their corresponding Group IIA elements). Also the decrease in solubility may be due to decrease ionic character of the hydroxides from Group IA to Group IIA
NB; A Suspension of slaked lime(calcium hydroxide) in water is called Milk of lime
CHEMICAL PROPERTIES
1.
ACTION WITH ACIDS AND ALKALIS
- Beryllium hydroxide is amphoteric. It reacts with excess sodium hydroxide forming a solution of sodium beryllate
edu.uptymez.com
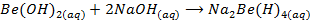
Sodium beryllate
The other hydroxide of group IIA metals do not react with alkalis but react with acids to form salt and water only
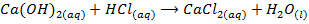
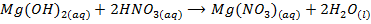
2.
ACTIONS WITH CARBON DIOXIDE
- Moist hydroxides absorb CO2 from air forming carbonates
edu.uptymez.com
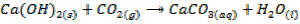
– When is bubbled through lime water (Ca(OH)2) white precipitate of CaCO3 are formed. This causes the lime water to turn milky. The milky colour disappears when excess CO2 in bubbled through it. The milky colour disappears because calcium carbonates is converted into calcium hydrogen carbonate which is soluble in water
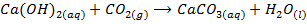
Clean solution White precipitate (milky)
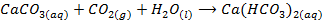
(Milky) (Excess) Clear solution
3. ACTION OF HEAT
The temperature at which the hydroxides begin to decompose increases down the group from about 3000C for beryllium hydroxide and magnesium hydroxide to about 7000C for barium hydroxide.
Example
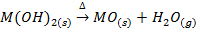
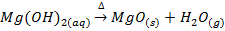
4. ACTION WITH AMMONIUM SALTS
All the hydroxides except Be(OH) 2 react with aqueous ammonium salts to give ammonia gas. The ammonia gas is easily identified because it turn alkaline to litmus paper.
Example
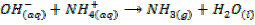
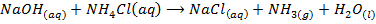
5. ACTION WITH SULPHUDIOXIDE
Sulphur dioxide turn lime water milky due to calcium sulphite formed. When excess SO2 is added the milky colour disappears (example, a clear solution is formed). The milky colour disappears due to the formation of calcium bisulphite which is soluble in water.
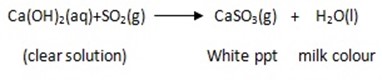
USES
1. Lime water is used to test for carbon dioxide.
2. A suspension of Magnesium hydroxide in water (milk of magnesium)is used as an ant-acid.
3. Ca(OH)2 is used in making builders mortar (mixture of slaked lime, sand and water).
4. A mixture of Ca(OH)2 is used in making bleaching powder.
5. Ca(OH)2 is used for neutralizing acids in the soil.
6. A mixture Ca(OH)2 and water (white wash) is used for coating walls and ceiling.
7. Ca(OH)2 is used in water softening.
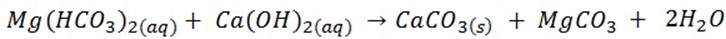
8. Ca(OH2) is used in sugar refining filtered.