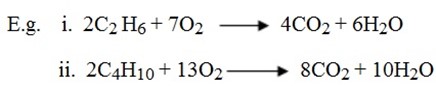ALIPHATIC HYDROCARBONS:
Nomenclature:
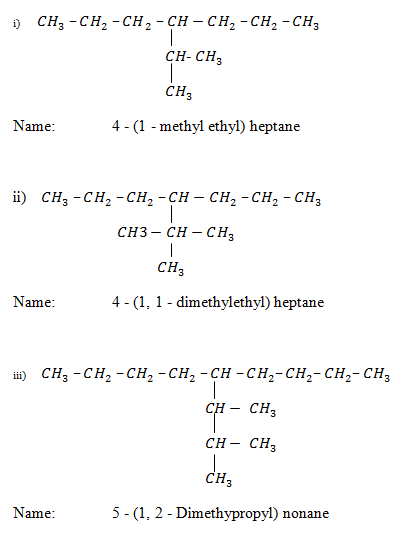
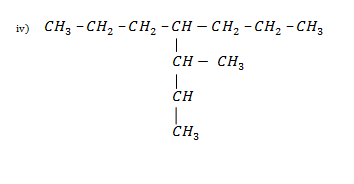
METHODS OF PREPARATION OF ALKANES
(a) Hydrogenation of Alkenes and Alkynes
Alkenes and alkynes react with hydrogen in presence of catalyst eg Ni/Pt around 200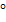 c or 300
c or 300 c
c
From Alkenes
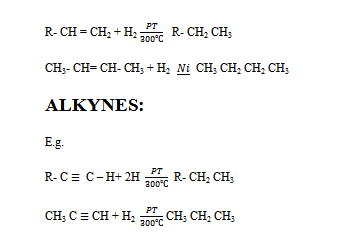
(b) Method 2 of preparation
Alkyl halide (haloalkanes)
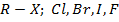
a) Reduction of alkyl halide by metal and acid
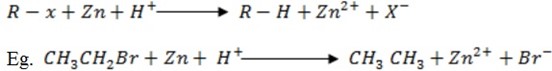
The hydrogen displaces Bromine. Only done in the presence of Zn metal
b) Reduction of Alkyl halide by using zinc and copper coupled with alcohol
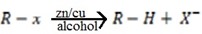
Note:
Both zinc and copper must be present together with alcohol.
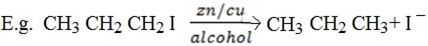
c) Reduction of alkyl halide by using hydroiodic acid in the presence of red phosphorus
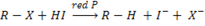
NOTE:
Function of red phosphorus is removing iodide so that hydrogen can react with the alkyl halide.
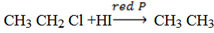
(c) Wurtz Synthesis
Alkyl halide in dry either solution react with sodium to produce alkane always the product has twice the number of carbon as that of alkyl halide.
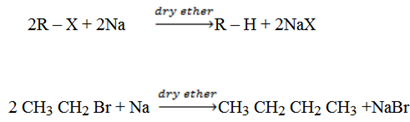
ii) 
NOTE:
Dry ether is very important condition.
(d) Decarboxilation of sodium carboxilate salt
This is the reaction between carboxylic salt and sodium hydroxide in the presence of calcium oxide. The product will have 1carbon less than the reactant.
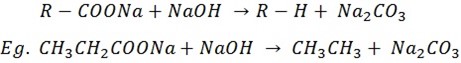
Exercise (H/W)
1. Preparation of alkanes from petroleum, coal, natural gas.
2. Read on method of preparation known as cracking. Preparation of alkanes from petroleum and natural gas.
– Petroleum is formed from the remains of tiny marine organisms that died and sank to the bottom of the sea millions of years ago.
– Petroleum is a mixture of many organic compounds, since the organic compounds are lighter than both the rock and the water they move upwards through the adjacent rock. Sometimes the organic Compound are trapped in porous rocks that are called reservoirs beneath impermeable rocks.
– Example of reservoir is limestone.
– Reservoirs from which petroleum can be extracted by drilling are referred to as oil fields.
– The petroleum obtained is referred to as a crude oil.
Fractional distillation of crude oil:
– Major components of crude oil are:-
(i) Residuals (coke, asphalt, tar).
(ii) Lubricating oils.
(iii) Fuel oils.
(iv) Diesels.
(v) Kerosene.
(vi) Naptha.
(vii) Petrol.
(viii) Petroleum gas.
The components of the crude oils are known as fractions and different fractions are separated by heating them in a process known as Fractional distillation and it is done in a distillation tower called a still.
The oil is first evaporated by heating . The vapour rises up and the tower acts as a giant heat exchanger removing heat from the gases as they rise up. Temperature falls to 20 by the time vapour reaches the top. The vapour condenses as they rise up.
by the time vapour reaches the top. The vapour condenses as they rise up.
The heavier ones i.e. those with higher boiling points condense first. Gaseous fractions pass out at the top.
Cracking
Some of the fractions obtained from the fractional distillation of the crude oil are converted into new products.
Cracking is the conversion of large molecules of organic compounds into compounds with smaller molecules.
There are two methods;-
(i) Thermal cracking.
(ii) Catalytic cracking.
In thermal cracking the large molecule organic compound is heated to a high temperature until its molecule break apart.
In catalytic cracking, a catalyst speeds up the cracking process.
(e) Preparation of Alkanes from alcohols:
By reduction of alcohols:
When alcohols are hated with concentrated hydroiodic acid and red phosphorus at 423k under high pressure, alcohols can be reduced to alkanes.
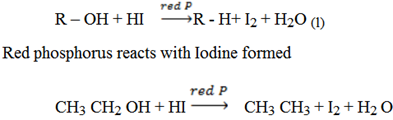
Physical properties of alkanes:
(a) Boiling point and melting point:
Alkanes have low melting point and boiling point.
Reason: Since alkanes are non-polar molecules with weak Van – der – Waal forces between them then low temperature is required to break the bond hence low melting and boiling point.
 Increase in molecular mass leading to the increase in melting & Boiling points
Increase in molecular mass leading to the increase in melting & Boiling points
NB:
If you compare straight chain and branched chain of same molecular mass; straight chain has higher M.P &B.P. Branched chained isomers have lower boiling points and melting point than straight chain isomer.
Reason: Branched chains are more compact hence have less surface area. This is why they have low M.P and B.P. Straight chains have higher surface area.
(b) Solubility:
They are soluble in non polar organic solvents but insoluble in polar compounds e.g. Water.
Chemical properties of alkanes:
In general, alkanes are non reactive (inert) compared to other classes of organic compounds.
Reason:
(i) They don’t have a functional group.
(ii) There bonds are quite strong i.e. c – c (strong bond). Large energy is needed to break the bond hence less reactive.
(iii) These two bounds are almost non polar and therefore neither electrophilic nor nucleophilic substitution reaction can take place. Can’t react with electron – loving species or a proton loving species (Nucleophilic).
– Electrophilic reacts with a negatively charged species.
– Nucleophilic reacts with a positively charged species.
Alkanes can undergo the following reaction;-
(i) Substitution reaction.
(ii) Oxidation reaction.
(iii) Thermal decomposition (cracking).
All these reactions take place at high temperature or under the presence of light energy.
I. SUBSTITUTION REACTIONS
a) Halogenations 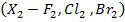
– This is addition of halogens.
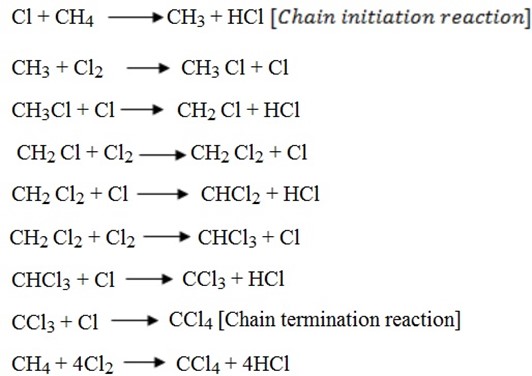
– The reaction between alkane and halogen is known as free radical substitution reaction.
– Free radical substitution reaction is the reaction in which a free radical substitutes atom/atoms in a molecule.
Q. What is a free radical?
A free radical is an atom or group of atoms which consist of unpaired electrons.
Example: 
Function of UV – light is to give out a free radical.
Mechanism of reaction

Homolytic sharing of electron i.e. equal sharing of electrons go to each chlorine atom
NOTE:
Free radicals are very reactive. It wants to become stable.
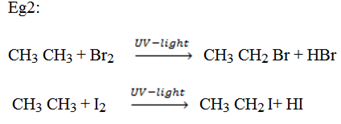
NOTE:
With fluorine, the reaction is violet and yield hydrogen fluoride and carbon.
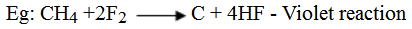
However, controlled fluorination in the presence of cobalt moderator the fluoral derivatives are formed.
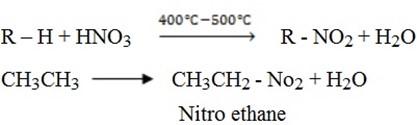
NITRATION (With Nitric acid)
This involves the substitution of hydrogen atom in alkane with NO2 group. This is done when a mixture of alkane and nitric acid vapour is heated at 400 c – 500
c – 500 c
c
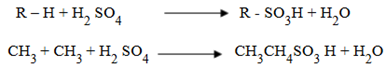
SULPHONATION (With sulphuric acid)
Alkanes when subjected to prolonged reaction with fuming sulphuric acid one hydrogen atom of alkane is replaced with – SO3 H group known as sulphonic group.

Oxidation Reactions:
When alkanes are ignited in the presence of excess oxygen they burn to form carbondioxide and water only.
Reaction is highly exothermic: