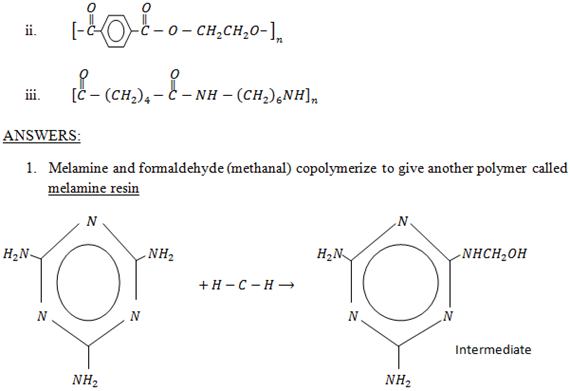
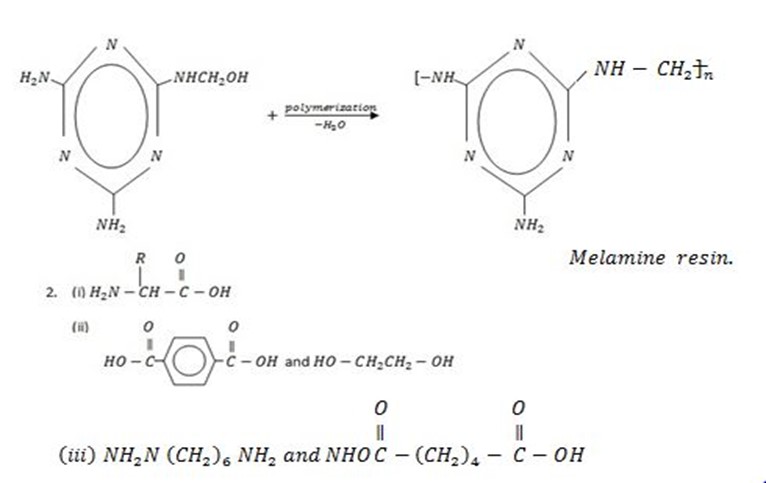
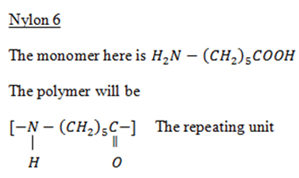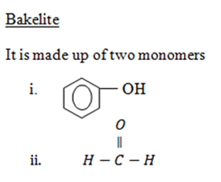It is obtained from 2 words
– Poly – many
– Merons- units
· Polymers are gigantic molecules which form chain of molecules.
·
A polymer is a giant chain like molecule obtained by intermolecular combination of large number of small molecules of the same or different types known as monomers.
E.g: Nylon 6,6
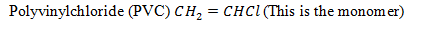
· Monomers are small molecules(simple molecules having low molecules) which capable of combining almost infinity together to form a polymers.
Structure of polymers
The primary structure of polymers is given by the types of monomers and their arrangement in molecules in the polymer.
If the polymer is made up of one types of monomer, it is known as Homopolymer or simple polymer.
E.g:
i. 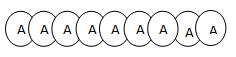
ii. PVC i.e 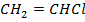
If the polymer is made up of two or more different types of monomers known as copolymer
There are 3 types of copolymer depending on order of polymer units
TYPES OF POLYMERS
- Synthetic Polymers.
- Natural / Synthetic rubber.
- Vulcanisation .
edu.uptymez.com
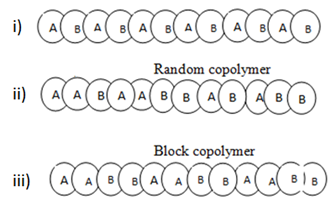
Examples of copolymer include:
(a) Nylon 6,6
(b) Polyester
Polymer can be classified into 2 classes on the basis of their structures
(a) Linear polymer
(b) Branched chain polymer
Linear chain polymer
Branched chain polymer
Properties:
i. Linear chain polymer have higher melting point and boiling point than branched polymers
Reason: – Van der Waal forces are stronger in the linear chain polymer.
ii. Linear polymer have higher density
iii. Linear polymer also have higher tensile strength
i)Classification based on molecular forces
There are 2 major classes
1. Thermosetting polymer
2. Thermoplastic polymer
THERMOSETTING POLYMER
– These are polymers which cannot be moulded on heating
– When heated they become hard.
Reason: – On heating they undergo further reaction which increases cross linked chain. They cannot be fused i.e infusible.
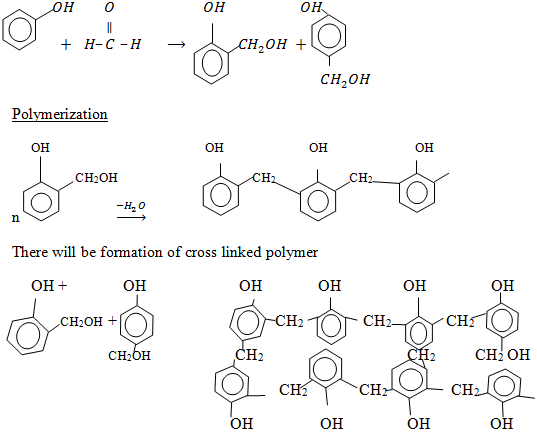
E.g: Bakelite
THERMOPLASTIC POLYMER
These are polymers which can be moulded on heating.
– They become soft when and when heated become stiff
E.g: PVC
They are linear structures with no cross linkage. Van der Waal will exist between molecules
ii)Classification based on the nature of polymer
There are 2 major classes
1. Natural
2. Artificial or synthetic polymer
Natural polymers
Example:
i. Cotton made up of cellulose, starch (made of glucose)
ii. Bamboo stick
iii. Paper
iv. Proteins
v. DNA, monomer nucleic acid
Artificial polymers
Example:
iii)Classification based on the mechanism of polymerization
There are 2 major classes of polymer
a. Addition polymer
b. Condensation polymer
A.
Addition polymerization
This is due to combination of unsaturated monomers (contain double or triple bond)
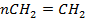
Ethene polyethene
Addition polymerization proceeds into 3 possible mechanisms
1. -Free radical polymerization
2. -Cationic polymerization
3. -Anionic polymerization
Free radical polymerization
Free radical polymerization is one which the reaction are catalysed by free radical obtained by organic peroxide (R–O–O–R`)
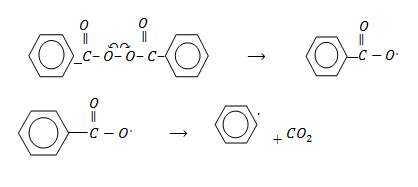
 Step: 01 Chain initiation step
Step: 01 Chain initiation step
.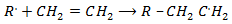
Step : 02 Chain propagation step
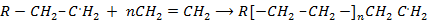
Step: 03 Chain termination step
The reaction stops when two free reactions combine or when the radicals undergo disproportionation
R 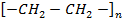
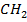
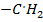 + R
+ R 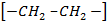
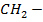
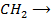
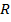
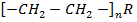
Example
Polymer
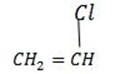
Chain initiation step .
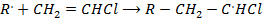
Chain propagation step
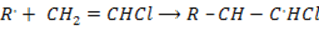
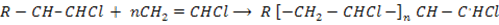
Chain termination step
R–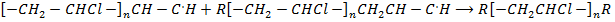
Polymer
Other polymer can be made from
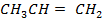
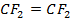
Cationic polymerization
In this polymerization the initiator is an electrophyl. The electrophyl adds to the alkene causing it to become a cation
Example of initiators:-
electrophiles H+, – NO2
i. Lewis acid e.g 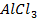 ,
, 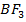 , H+
, H+
Mechanism
1. 1. Chain initiation step
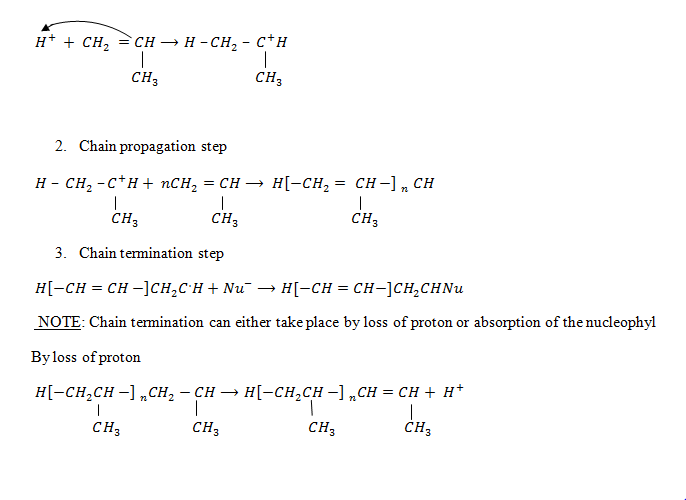
Here we use a strong base to remove the hydrogen
Other monomers which can undergo Cationic polymerization include

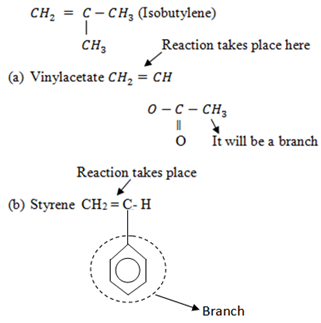
Using Isobutylene
1.
1. Chain initiation step
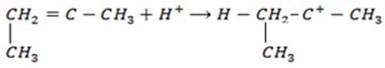
2.Chain propagation step
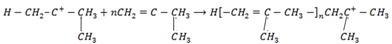
3.
3. Chain termination step
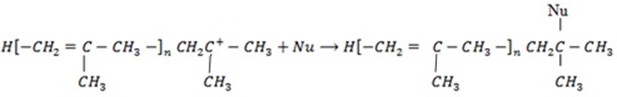
Anionic polymerization
Mechanism
Initiated by nucleophile that react with alkenes to form anionic propagating site
Example of nucleophile
NaNH2
Butyl lithium BuLi
R – O– (Alkoxide)
Grignard reagent
This reaction occur if the carbon is stabilized by a suitable electron withdrawing group
Mechanism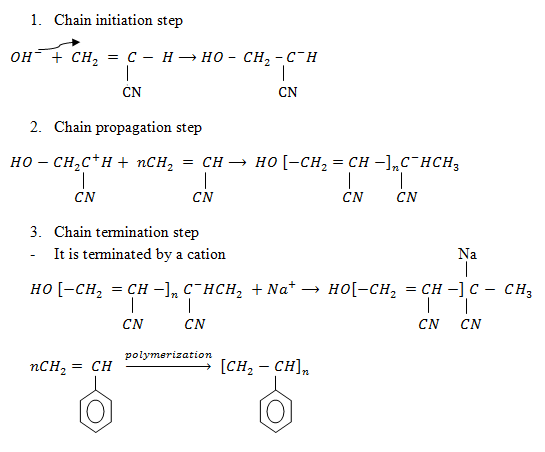
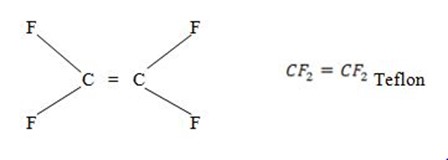
Polytetraloroethene

Uses of polymers :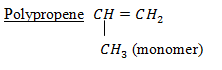
Used in making pipes, bags, container and unbreakable bottles
Polyethene 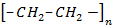
Used in making pipes, containers, bottles, electrical insulation
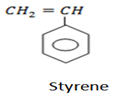
Styrene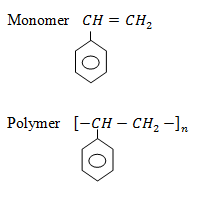
USES:
– Household goods
– Electrical insulation
– Optical lenses
Polychloroethane
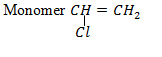
Typical chain section

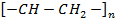
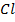
USES;
– Cable insulation
– Pipes
– Hoses
– Fabrics
– Flooring
Polytetrafluoroethane
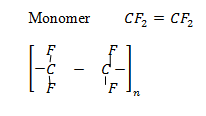
USES:
– -Surface coating of fans (non stick)
– -Pipes
– -Apparatus for chemical plants
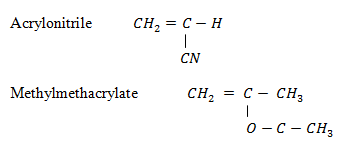
6. Polypropanonitrile
 Monomer
Monomer
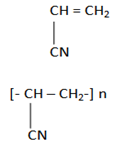
USES:
-Tertiles
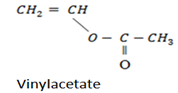
7.Polyethanylethanoate (vinyl acetate)

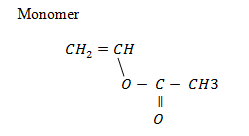
USES:
– -Paints
– -Adhesives
– -Water repellent coating
8.Perspex (polymethyl-2- methyl(propanoate)

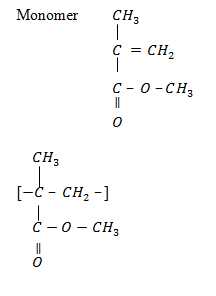
USES:
– -Optical lenses
– -Glasses
– -Used as glue and paint
CONDESATION POLYMERIZATION
It takes place through condensation reaction between two bifunctional or trifunctional monomers with simultaneous loss of small molecules such as H2O, HCl, NH3 etc.
Example:
i. Polyesters
ii. Polyamides or polypeptides
iii. Terylene
It is made up of two monomers
Polyester e.g Terylene.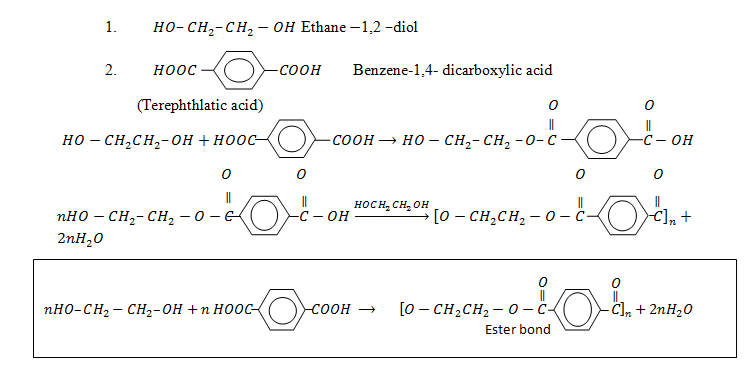
Polyamides e.g Nylon 6, 6 (polyamide)
It is made up of two monomers
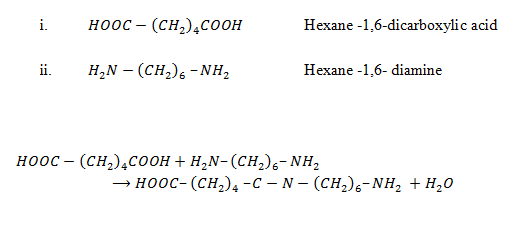
Nylon 6,6