Thermal decomposition (PYROLYSIS)
This is breaking down of higher alkane into lower alkane by heating alkanes in absence of air.
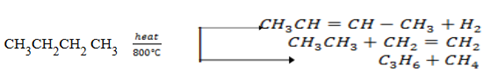
Alkane and alkene are the only possibilities.
NOTE:
No two alkenes will be formed.
Catalytic cracking (Isomerization)
When straight chains of alkanes are heated in aluminium chloride in the presence of dry hydrogen chloride at 300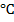 gives a branched chain isomers. In this process there is no breaking of the compound but it is changed to branched chain.
gives a branched chain isomers. In this process there is no breaking of the compound but it is changed to branched chain.
This process is used in petrol chemical industry. The branched chain alkane has higher octane number. Hence branched chain burns easily than straight chains.
Question:
– How to form aromatic compounds i.e. Aromatizations
– Uses of alkanes
Exercise:
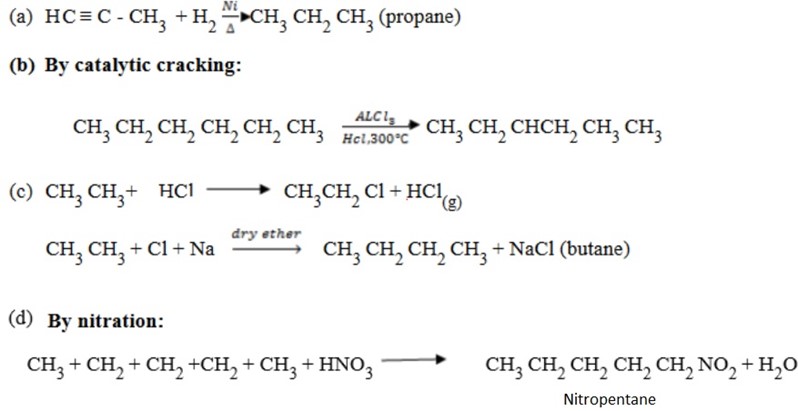
Qn. How can the following conversions be achieved?
(a) Propyne to propane
(b) Hexane to 3methylpentane
(c) Ethane to butane
(d) Pentane to nitropentane
Solution:
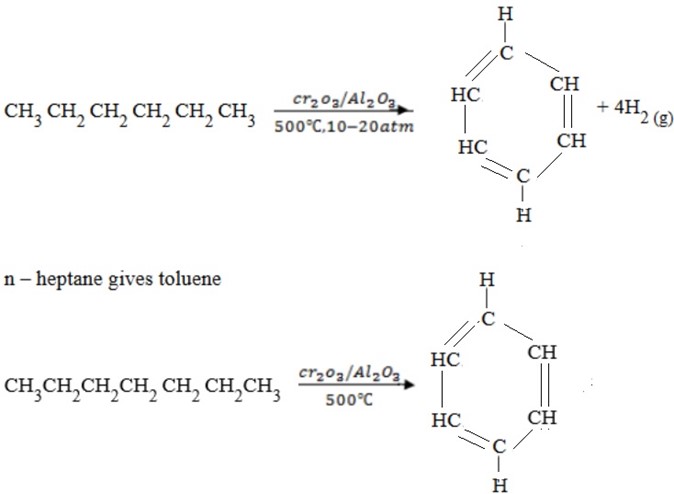
AROMATIZATION
– Alkanes containing six or more carbon atoms when heated under pressure in the presence of suitable catalyst get cyclised to give aromatic compounds.
E.g.
n – hexane gives benzene
USES OF ALKANES
Alkanes are the simplest organic compound containing carbon and hydrogen. Some major uses of these compounds are;
(i) Lower alkanes occurring as natural gas and lighter petroleum fractions are used as fuels.
(ii) Low – boiling liquid alkanes e.g. hexane are uses as solvents.
(iii) Heavy petroleum fractions are used as lubricants (grease) and for obtaining waxes and vaseline
(iv) The products of cracking process are generally used for producing linearalkyl benzene (LAB) used as a raw material for manufacturing detergents.
ALKENES
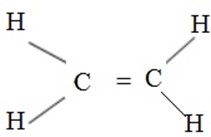
These are hydrocarbons which are unsaturated. They contain double bond between two carbon atoms.
Functional group is C = C and share the same formula with cycloalkanes (cyclalkanes) are known as fractional isomer.
General formula: Cn H2n
Cycloalkanes: Cn H2n
The type of hybridization is sp2 hybridization since it is trigonal pyramidal shape.
Nomenclature:
The first member is Ethane since we can’t have double bond in a single carbon atom.
E.g. C2 H4 – Ethene
C3H6 -Propene
Isomerism:
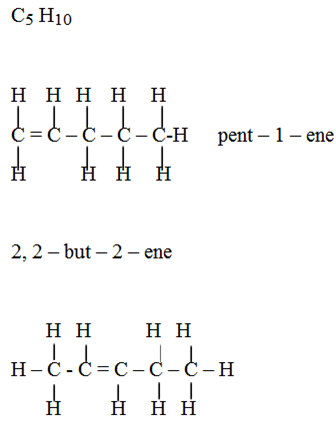
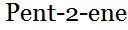
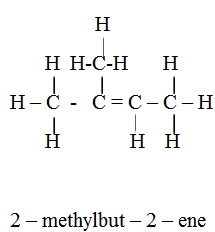
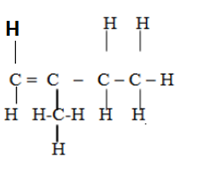
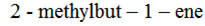
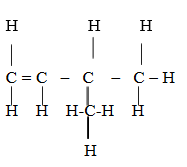
3 – methylbut -1- ene
Naming of different compounds:
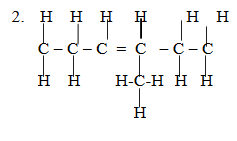
3 – propylhex – 2 – ene
Functional isomers;- They have the same general formula but different functional group. Alkenes and cyloalkanes both have general formula Cn H2n
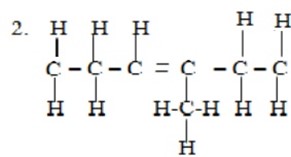
3 – menthlyhex – 3 – ene
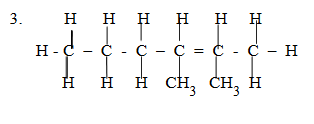
2, 3- dimethyl hex-2-ene
4. 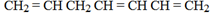
If more than 1 double bond, add a prefix (a) to hept
6 – methylhepta – 1, 3, 6 – triene
Types of Isomerism in alkenes:
Alkenes show 4 types of isomerism;
(i) Chain isomerism
(ii) Positional isomerism
(iii) Geometrical isomerism
(iv) Functional isomerism
Chain isomerism: (Skeletal isomerism)
This is due to the difference in the structure of carbon chain.
Example
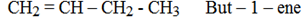
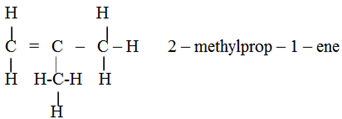
Positional isomerism:
This arises from the difference in the position of the double bond.
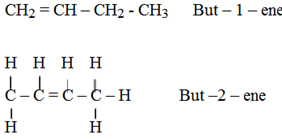
Geometrical isomerism:
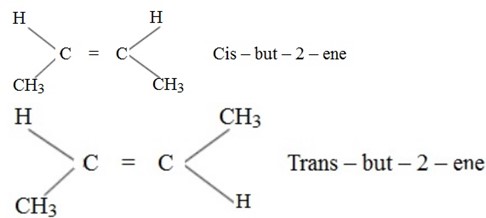
Definition:
– Is brought in the difference in the spatial arrangement of atoms or group of atoms about the double bond.
Functional isomers:
These compounds have the same general formula but difference functional group.
E.g. Alkene and cycloalkanes i.e Cn H2n

Cyclobutane

Cyclobutene
Laboratory preparation of Alkenes:
(i) Dehydrohalogenation of haloalkanes (alkyl halides)
– There is elimination reaction.
– The reaction is done in alcoholic basic medium.
Note:
General formula for alky halides is R – X, X = Cl, Br, F
– When alky halide is heated with alcoholic solution of sodium hydroxide or potassium hydroxide, hydrogen and halogen will be eliminated and alkene is formed.
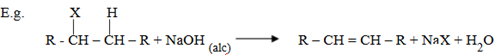
This is known as Basic induced elimination reaction
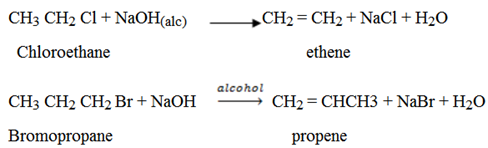
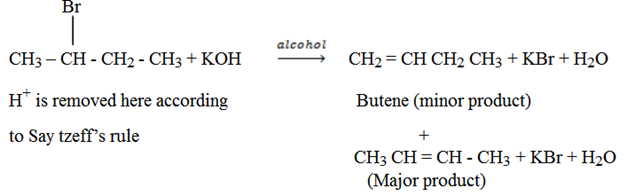
SAYTZEFF’S RULE
It states, “during elimination reaction, the electrophyl (H+) is removed from carbon atom with fewer number of hydrogen atom.
Example of reactions which apply Saytzeff’s rule;-
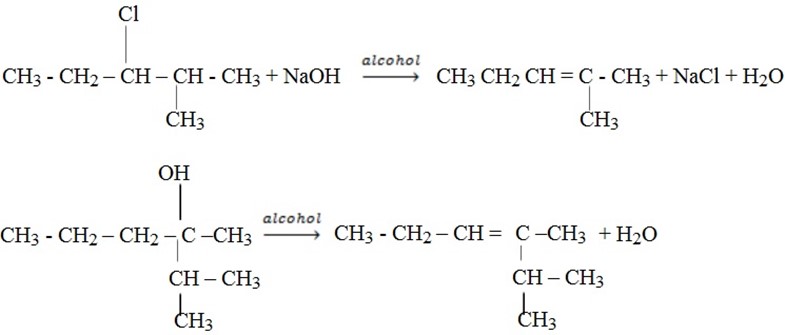
(ii) (a) Dehydration of alcohol
This is done using concentrated sulphuric acid by warming about 175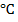 – 180
– 180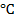 . You react alcohol with conc. sulphuric acid.
. You react alcohol with conc. sulphuric acid.
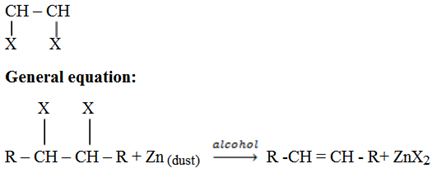
Note:
Temperature is very important 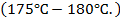 this reaction is sensitive to temperature
this reaction is sensitive to temperature
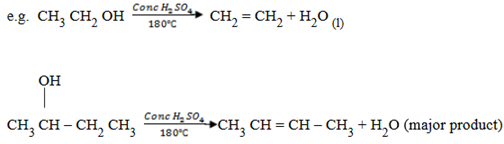
NOTE:
H3PO4 (conc.) can be used as a dehydrating agent (373 – 383k)
(b) Dehydration by passing the vapour of alcohol over aluminium oxide (alumina) at 350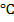
iii) Dehalogenation of vicinal dihalides
Vicinal means the halogens are on the adjacent carbon of the same carbon.
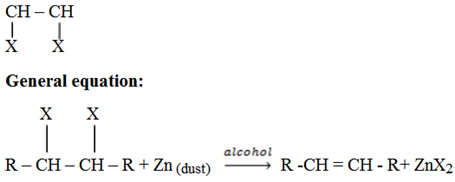
iv) (a) Controlled hydrogenation of alkynes
It is done under palladium catalyst which is poisoned by calcium carbonate and quinoline and this reagent is known as Lindlers catalyst.
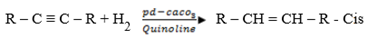
Note:
Poisoned means that the palladium is not pure
(b) Sodium, Lithium and Ammonia
This is not complete hydrogenation.
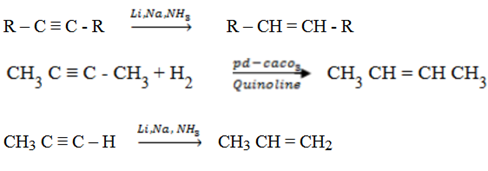
Chemical properties of Alkenes:
NOTE: Alkenes are more reactive than alkenes due to the presence of pi bond which is relatively weak. Hence, can react easily
1. Addition of hydrogen halides (HX)
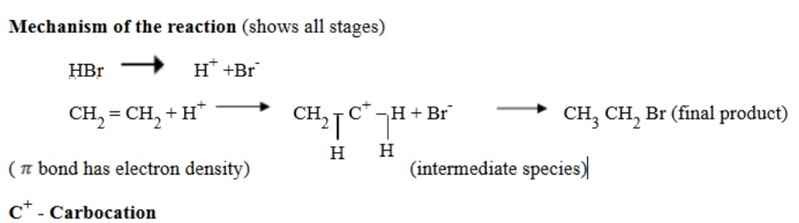
Alkenes undergo electrophilic addition reaction (electron loving/electron deficient).
Electrophilic addition reaction is the reaction in which electrophyl is added first followed by nucleophyl.
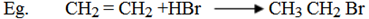
Addition reaction of 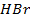 follows a rule known as Mark KovniKov’s rule
follows a rule known as Mark KovniKov’s rule
Mark KovniKov’s rule:
It states, ‘During the addition reaction the electrophyl (hydrogen) is added to carbon atom with more number of hydrogen atoms’.
Eg: 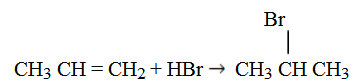
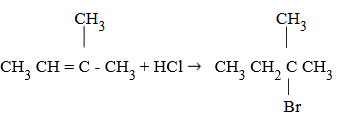
Why Mark Kovni Kov’s rule?
It is because of the formation of stable carbocation. Hence the rule is used so as to form a stable carbocation.
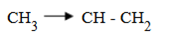
NOTE:
Stability of carbocation is due to the supply of electron from the alkyl group as shown above. The halogen is added to the stable carbon.
In long chains, a stable carbocation will be formed when the carbon is bounded by many alkyl groups (since the alkyl group will be supply electrons
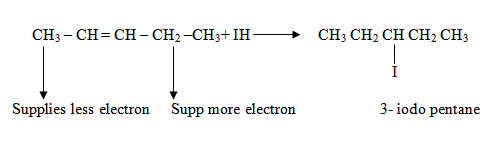
Note:
Hydrogen is added to the more stable carbon.
2. Hydration of alkenes:
Hydration means addition of water.
– This is addition of water in the presence of mineral acids. The most preferred acid is conc H2SO4. The mixture should be heated in order to form alcohol.
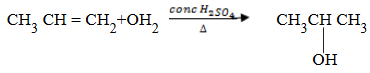
Carbocation will be formed in CH