DIRECTING EFFECT
Carbons in benzene with only one substituent group can be formed as follow;
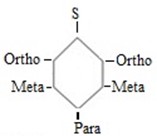
The subustituent is activated, then it tends to direct incoming electrophile substituent at Ortho and para position i.e All activators are Ortho – para directors.
This can be explained considering;
i.Position of carbonium ion.
ii.Stability of intermediate carbonium ion.
I. POSITION OF CARBONIUM ION
Understand this consider mesomerism of phenol in which OH– activator is directly attached to benzene ring.
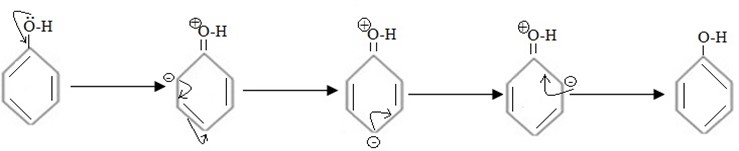
Above mesomerism (+M). It can be seen that despite the fact OH – (activator ) increase electron density through out the benzene ortho, para positions are more effected and hence ortho and para, carbons become better site for incoming electrophile.
II. STABILITY OF INTERMEDIATE CARBONIUM ION
1st CASE
Incoming electrophile attach at ortho position. Consider electrophilic substitution reaction in aniline
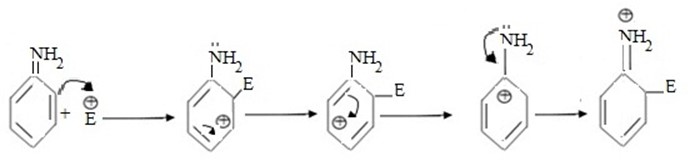
Mecomerism it is clearly understood that intermediate carbonium ion is stabilised by lone pair electrons in nitrogen of amino group (-NH2) and hence it is more stable
2nd CASE
If incoming electrophile attaches (substitute) at meta position consider the same reaction in aniline.
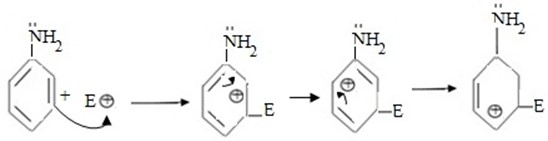
- In this case intermediate carbonium is not stabilised by lone electrons of nitrogen in amino group and hence it is less stable.
edu.uptymez.com
3rd CASE
If incoming electrophile substituents are at para position.
. Consider the same reaction in aniline.
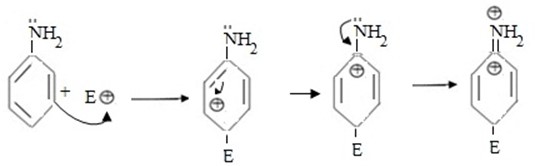
· . In this case carbonium ion is stabilised by lone pair of nitrogen in group amino hence it is more stable.
CONCLUSION
Since carbonium ions formed in 1st and 3rd case are more stable than that formed in 2nd case. Ortho and para positions are prefered sites for incoming electrophile.
NOTE:
- Alkyl group act as ortho-para directon by doing partial neutralization of positive charge formed on the adjustment carbon
edu.uptymez.com
(The partial neutralization is done by positive inductive effect exerted by alkyl groups). Hence ortho – para directing of alkyl groups is simply explained by considering stability intermediate carbonium ion like in (ii) above.
i.e.
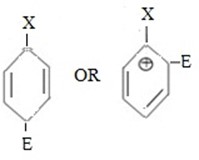
Are ortho – para directors due to stability intermediate carbonium ion. This is simply because despite the fact that lone pairs in halogens have not good participation in mesomerism for reason which has explained, but in presence of positive charge on adjacent carbon lone pair electrons participate in neutralizing positive charge on the carbon.
i.e.
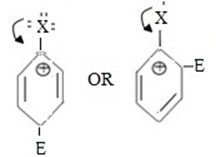
- Among the two products (ortho product and para product in most cases para product is major product why?
edu.uptymez.com
Reason
Due to steric hinderance exerted by the substituent originally present in benzene, ortho carbons which are closer to the substituents experience the effect strongly and hence incoming electrophile is more favoured to substitute at para carbon which is far from the substituent.
But if the substituent in halogen, ortho product become major product why?
Reason
Halogens like Cl have very small atomic size, Thus they exert very small steric hinderance thus make incoming electrophile to substitute first at ortho carbons (for every two ortho) carbons there is only one para carbon).
Deactivators with exceptional of halogens directs incoming eletrophile at meta position i.e. Deactivator (with exceptional halogens) are meta directors.
This can be explained by considering
i. Position of carbonium ion
ii. Stability of intermediate carbonium ion.
I. POSITION OF CARBONIUM ION
- To understand this consider mesomerism (-M) in benzoic acid
edu.uptymez.com
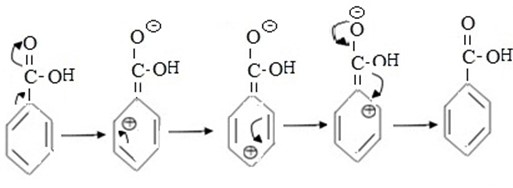
From the above shown mesomerism it can be seen that despite the fact that carboxylic group (-COOH) deactivate the whole benzene ring ortho and para positions are more effected and hence meta carbon somehow become pereferd position for incoming eletrophile.
II. STABILITY OF INTERMEDIATE CARBONIUM ION
- Consider the electrophilic substitution reactions in benzoic acid.
edu.uptymez.com
1st CASE
If incoming electrophile substitute of ortho position.
i.e.
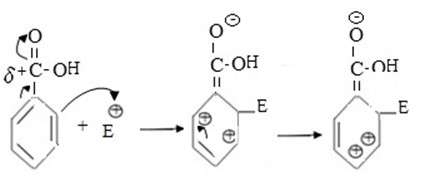
- Intermediate carbonium is not stable as result of very large repulsion force between closer positively charged ions in adjacent carbons.
edu.uptymez.com
2nd CASE
If incoming eletrophile substitute of meta position.
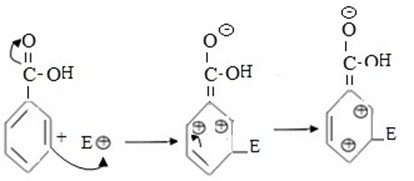
- Carbonium ion formed in this case is somehow more stable as a result of comparable small repulsion force between position charged carbons which are not adjacent.
edu.uptymez.com
3rd CASE
If incoming electrophilic substitutes at para position. Also Intermediate carbonium ion formed is not stable as a result of very large repulsion force between closer positively charged ions in adjacent carbons.
i.e.
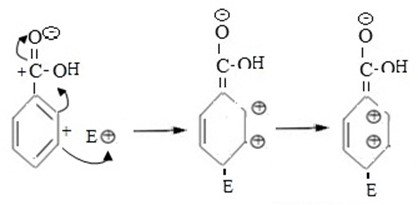
CONCLUSION
Intermediate carbonium ion formed in second case is more stable than in 1st case and 3rd case and hence meta position is better site for incoming electrophile.
SUMMARY ON DIRECTING EFFECT
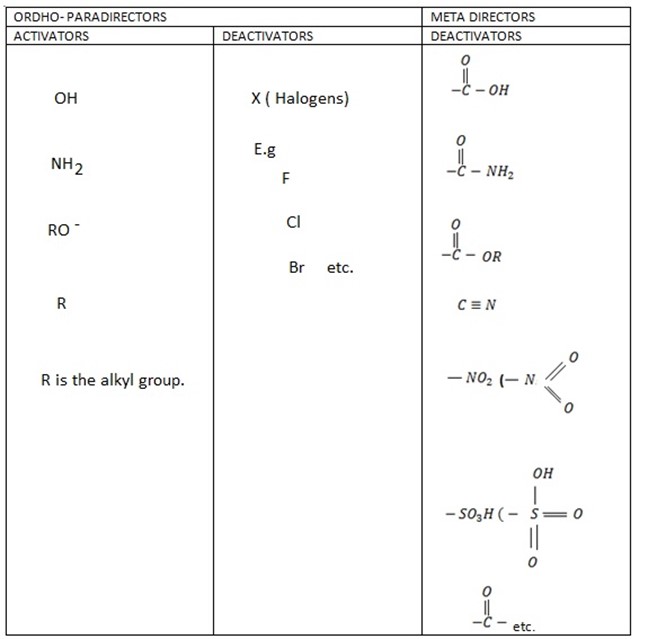
SOLVED PROBLEMS
QN 1. Arrange the following compounds in order of reactivity towards.
i. Nucleophile.
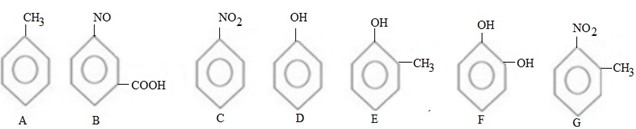
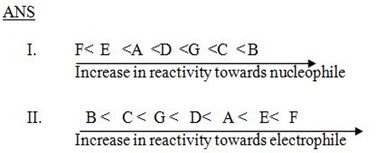
Qn. 2. Explain why alkylation of nitrobenzene is much slaver that of methy l benzene?
ANS
Alykylation in given compounds is electrophile substitution reaction so presence of nitro group which is an electron withdrawing (deactivator) in nitrobenzene deactivate. Its reaction towards electrophile while presence of methyl group which is electron receptor group (activator) in methyl benzene activate its reaction towards electrophile and hence alkylation of nitrobenzene become less than that of methyl benzene.
Qn. 03. Complete the following organic reactions.
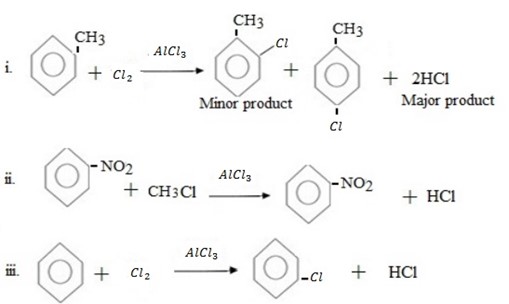
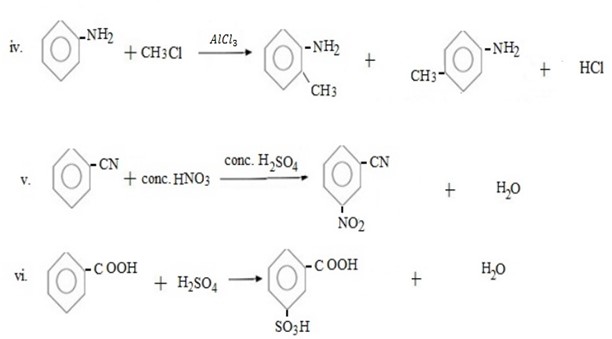
Qn. 04. NECTA 1994
Write structural formula of main substitutional product in the following organic reactions.
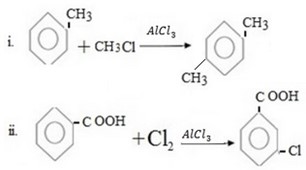
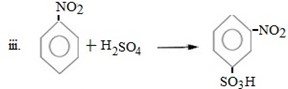
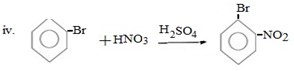
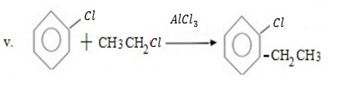
Qn 05. NECTA 1993
Which substituent entered first in the following organic compounds giving reasons.
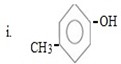
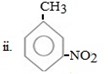
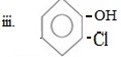
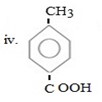
ANS
i. Either of the two substituents entered first.
Reason
In given compound OH and CH3 are para related and OH– and CH3 are ortho –para directors forming para product as a major product and hence either of the two entered first so as to direct incoming substituent at para position.
ii. NO2 entered first
Reason
In given compound CH3 and NO2 are meta related so being meta director it must be entered first so as to direct the incoming CH3 group at meta position.
iii.Cl entered first
Reason
In given compound OH and Cl are ortho related and OH and Cl are orth-para directors, OH– forming product a major product (as result of its large steric hinderance while Cl form ortho product as major product (as result low steric hinderance ) and Cl– must be entered first so direct at ortho position.
Reason
In given compound CH3 and COOH are para related, CH3 being ortho –para product forming para product as major product must be entered first so as to direct incoming – COOH at para position.
Qn 6.
Show how the following conversions can be achieved.
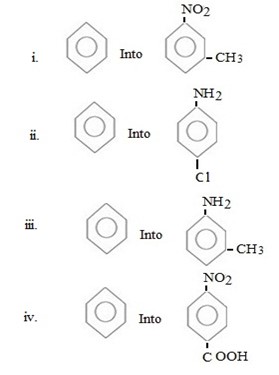
ANS
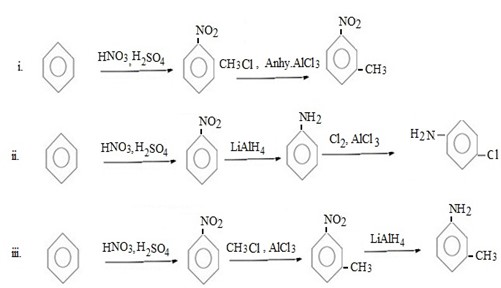
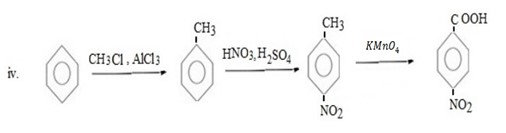
FURTHER CHEMICAL REACTIONS OF BENZENE
Apart from electrophilic substitution reactions benzene can undergo the following reactions.
i. ADDITION REACTIONS
- Under vigorous condition benzene can undergo addition reaction.
edu.uptymez.com
eg.
(a) HYDROGENATION
Benzene can react with hydrogen under presence of nickel or platinum catalyst yielding cyclohexane.
i.e.
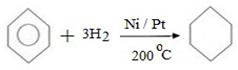
(b) CHLORINATION
- Under presence of U.V a very high temperature benzene react with chlorine to give 1,2,3,4,5,6-hexachlorocyclohexane.
edu.uptymez.com
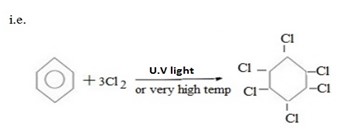
TOLUENE (METHYL BENZENE)
- Toluene is the aromatic compound which is formed when one halogen atom of benzene is replaced by methyl group.
edu.uptymez.com
i.e. Structure of toluene is ;-
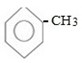
PREPARATION OF TOLUENE
(a) METHYLATION OF BENZENE
Generally;
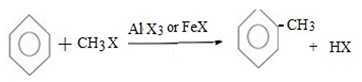
Example:
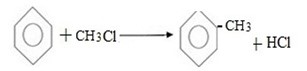
( b) Reaction between halobenzene and halomethane under;
Presence of sodium and dry ether.
Generally.
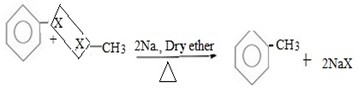
Example;
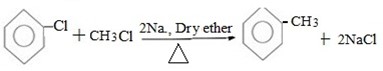
PHYSICAL PROPERTICES OF TOLUENE
- It is more denser than water.
- It is solube in non-polar solvents like organic solvent (Toluene itself is good organic solvent)
- It melts at temperature of -950C and boils at 1110C
- Its vapour density is large than that of the air
- It is colourless liquid at room temperature.
edu.uptymez.com
In most cases toluene is used as organic solvent instead of benzene because it is less toxic.
CHEMICAL REACTION OF TOLUENE
Commonly toluene undergo the following chemical reactions
i. Side chain chemical reactions
ii.Electrophilic substitutions in benzene ring
I. SIDE CHAIN CHEMICAL REACTIONS
Under this heading toluene undergo the following
a)Oxidation
b)Free radical substitution reactions.
A) OXIDATION
With milder oxidizing like MnO2 agent benzaldehyde is med.
i.e
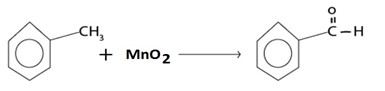
But with strong oxidizing agent like kmno4 and K2Cr2O7 ie acid is formed
eg.
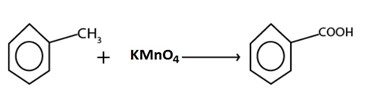
B) FREE RADICAL SUBSTITUTION REACTION
· With halogens under presence of U.V or very high temperature tends to undergo side chain radical substitution reactions.
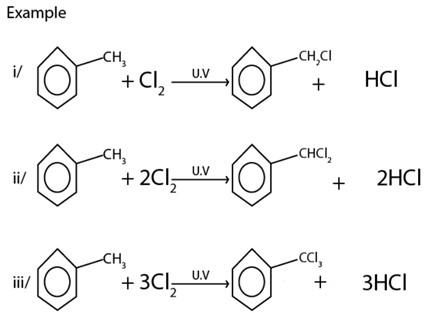
II. ELECTROPHILIC SUBSTITUTIONS IN BENZENE RING
Consider this heading toluene undergo similar chemical reaction as those of benzene. The only difference is that methyl group in toluene act as ortho director forming para product as major product
Example of electrophilic substitution reactions of Toluene
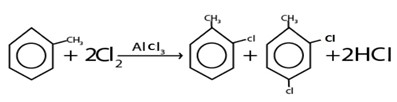
i. HALOGENATION
Generally
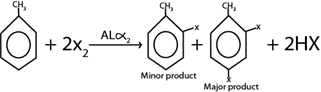
Example
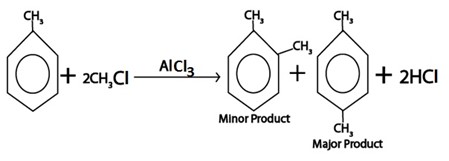
ii. ALKYLATION
Generally
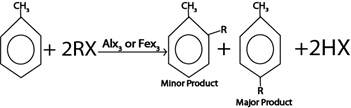
Example
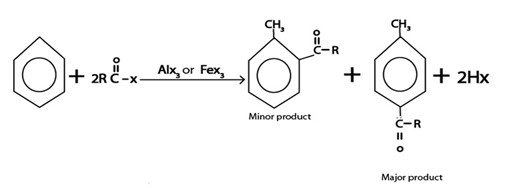
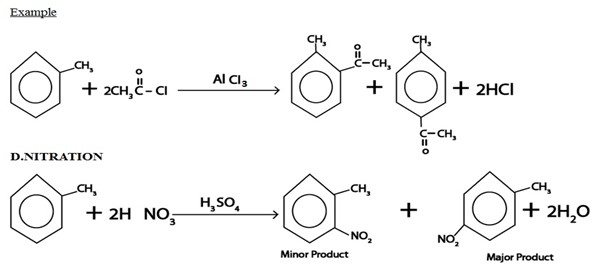
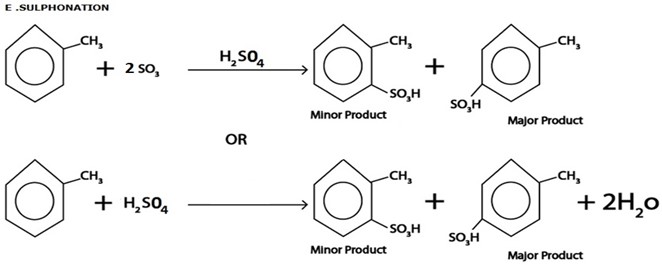
iii. ACYLATION
Generally
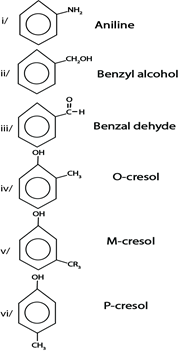
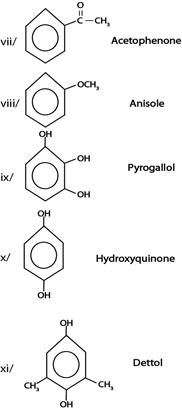
SOME STRUCTURE OF AROMATIC COMPOUNDS $ THEIR COMMON NAME
II. SECONDARY (20) HALOALKANE
These are haloalkanes where by a carbon with halogen is directly bonded to two alkyl groups
Thus for haloalkane with only one halogen the carbon with halogen is also directly bonded to only one hydrogen atom.
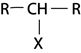
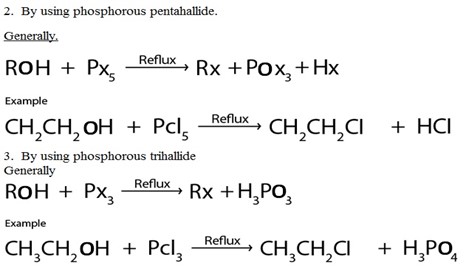
III. TERTIARY (3O) HALOALKANES
These are haloalkanes where by a carbon containing halogen directly bonded to three alkyl groups.
Thus in tertiary haloalkanes there is no hydrogen atom which is directly bonded to carbon with halogen.
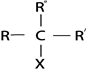
Haloalkanes are named by naming halogen as substituent of alkanes.
PREPARATIONS OF HALOALKANES
a). FREE RADICAL SUBSTITUTION REACTION OF ALKANES
Generally.
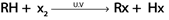
Example.
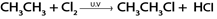
In above reaction chlorine must be present in limited amount so as to prevent further chlorination of the product otherwise
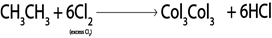
b). HALOGENATION OF ALKANES
Generally
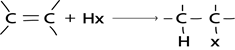
Example
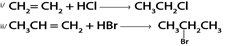
C). HALOGENATION OF ALCOHOL
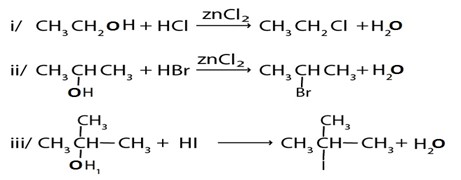
1. By using hydrogen halide
Alcohol reacts with hydrogen halide to give haloalkanes
Example
2. By using phosphorous pentahallide.
Generally.
4. Reaction by using thionylchloride
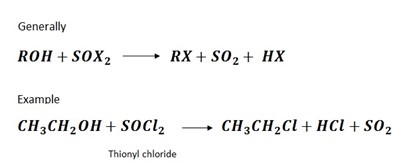
PHYSICAL PROPERTIES OF HALOALKANES
Melting and boiling point of haloalkane are greater than those of corresponding members of alkanes(alkane whose moleculer mass do not differ with those of haloalkanes) as a result of highly polarity of C-X bond
eg Cδ+ — Clδ-
Haloalkanes are soluble in organic solvent (haloalkanes themselves are good organic solvent i.e they tend to form miscible with another organic solvent) but are almost insoluble in water .
CHEMICAL REACTIONS OF HALOALKANES.
Haloalkanes undergo the following chemical reactions
a) Nucleophilic reaction
b) Elimination reaction
c) Reaction which leads to formation of Grignard reagent
d) Wurtz/coupling/fillings reactions
e) Reduction
A) NUCLEOPHILIC SUBSTITUTION REACTION
Nucleophilic substitution reaction in Haloalkane undergo two types of mechanism namely:
i) SN mechanism
ii) SN2 mechanism
i. SN. MECHANISM
This is nucleophilic substitution reaction where by there is only one molecule which is involved in rate determining
Definition of Rate dertermining step
This is the lowest step in reaction mechanism
SN. Mechanism is more common in tertiary haloalkanes as a result of high stability brought by strong positive active effect exerted by three alkyl groups
Illustration.
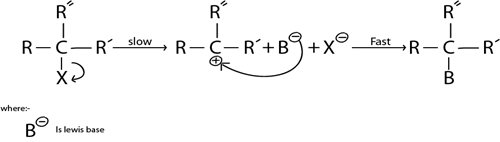
ii. SN2 MECHANISM
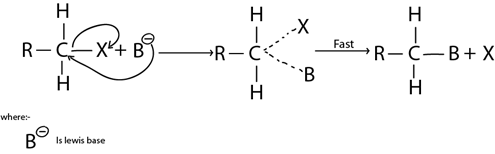
This is the Nucleophilic substitution reaction mechanism which by there are two molecules in rate determining step.
It is more common in primary haloalkanes (also in secondary haloalkanes)
Generally reactivity of haloalkanes towards nucleophile follow the following
