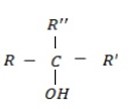Haloalkanes
i. Formation of alcohol
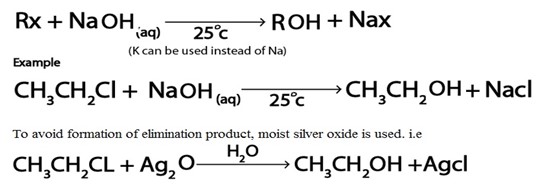
Haloalkanes reacts with alkaline solution like NaOH(aq) yielding alcohol
Generally
ii. Formation of amines.
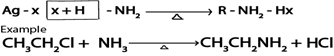
Haloalkanes react with ammonia yielding amines
Generally.
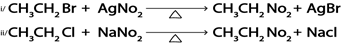
iii. Formation of nitroalkanes
Generally
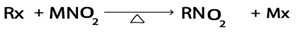
Where:
M is g, Na, k e.t.c
Example
iv. Formation of Ester
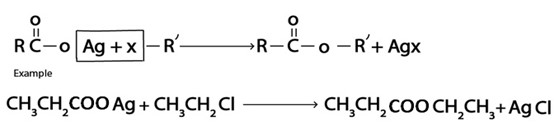
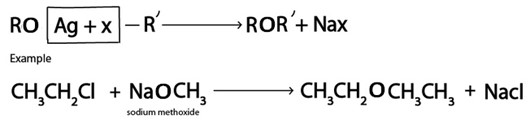
V. Formation of Ether
Generally.
vi. Formation of Nitrile
Generally

Nitrile is used to synthesize various organic compound
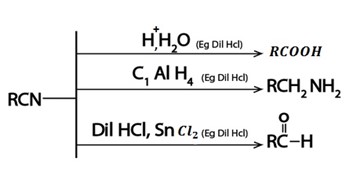
So the reaction (formation of nitrile) is very important in dealing with conversion problems which show that number of carbons have increase by one.
Example
Show how the following conversion can be
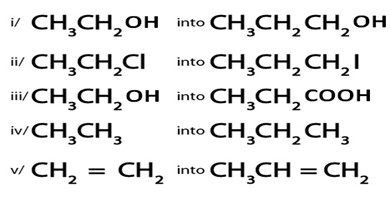
ANS.
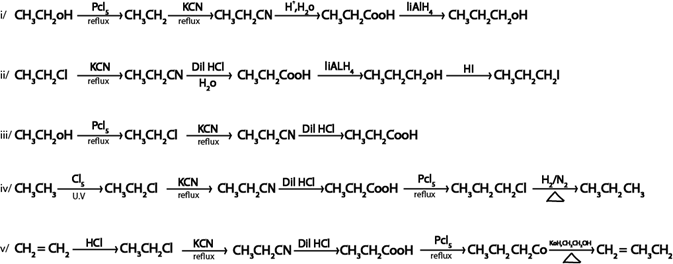
Replacement by another halogen
- Halogen in haloalkane can be replaced by another halogen which is more nucleophilic.
- The strength of nucleophilic character of halogen follow the following order.
edu.uptymez.com
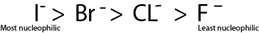
B). ELIMINATION REACTIONS.
Elimination and nucleophilic substitution reactions in haloalkane are competitive reactions.
Conditions which favour elimination reactions are
– The reaction should under taken in non-aqueous solution (in this case the reaction is undertaken inpresence of alcohol)
– Presence of strong and concentrated base like conc. KOH
– The reaction should be undertaken at hight temperature
– Tertiary haloalkane
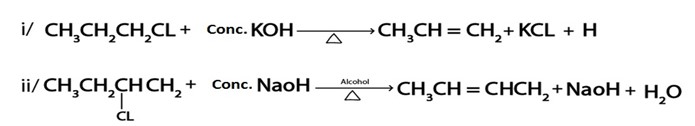
Formation of major product in elimination reaction (if there is ability of forming more than one product ) is governed by saytzeff’s rule which. States that. “The alkane with great number of alkyl group is more stable.
– So according to saytzeff’s rule if there is possibility of forming more than one elimination product, the major product is the alkene with greater number of alkyl groups
· Elimination product of
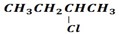 is ether
is ether
i. CH3CH=CHCH3 or
ii. CH3CH2CH=CH2, so according to saytzeff’s rule (i) is Major PRODUCT
·There are two types of mechanism of elimination reaction in haloalkane namely
a) E1 mechanism
b) E2 mechanism
A. E1 MECHANISM
·This is mechanism of elimination reaction where by there is only one molecule which is involved in rate determining step
– It is more common in tertiary haloalkane as result of high stability of intermediate carbonium ion which is brought by very strong positive inductive effect from three alkyl groups.
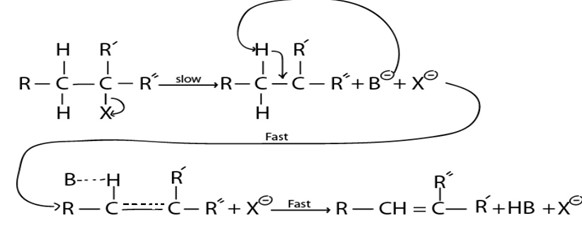
B. E2 MECHANISM
·This is the elimination reaction mechanism where by there are two molecules which are involved in rate determining step.
– It is more common in primary haloalkane (also in secondary haloalkane) due to instability of intermediate carbonium ion which could be formed if it undergo mechanism as a result of weak +I exerted by one alkyl group.
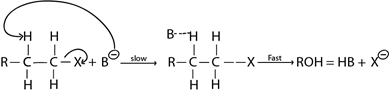
Examples of elimination reaction in haloalkanes are
NOTE:
Possibility of haloalkanes to undergo elimination reactions follow the following trend
Tertiary haloalkanes>Secondary haloalkanes > Primary haloalkanes
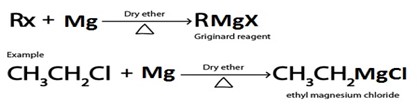
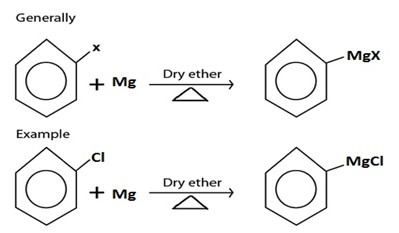
C. GRIGINARD REAGENT FORMATION
· Grignard reagent is alkylmagnesium halide (RMgx)
· Haloalkane react with magnesium under presence of dry ether yielding Grignard reagent.
Grignard reagent.
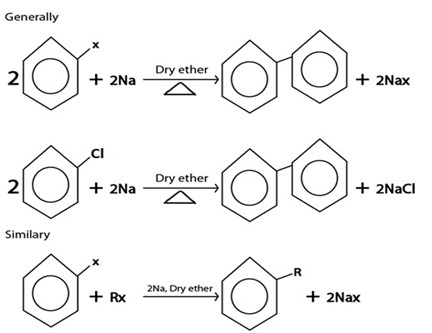
D. WURTZ REACTION.
Generally
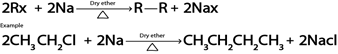
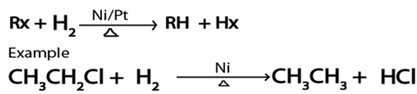
E. REDUCTION.
· With hydrogen gas under presence of nickel or plantinum catalystal heat alkananes formed from haloalkanes.
Generally
HALOARENE
This is the halohygrocarbon which is formed when atleast one hydrogenation of benzene is replaced by halogen.
– The simplest one is formed when only one hydrogen atom of benzene is replace by halogen.
i.e
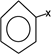
Chemical reactions of haloarenes include.
i. Nucleophilic substitution reaction
ii. Grignard reagent formation
iii. Wurtz/Fitting/coupling reaction.
iv. Reduction
v. Electrophilic substitution reaction
I. NUCLEOPHILIC SUBSTITUTION REACTION
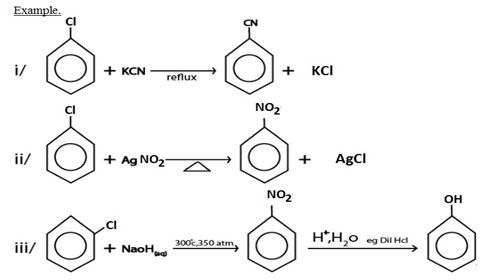
II. GRIGINARD REAGENT FORMATION
III. WURTZ REACTION
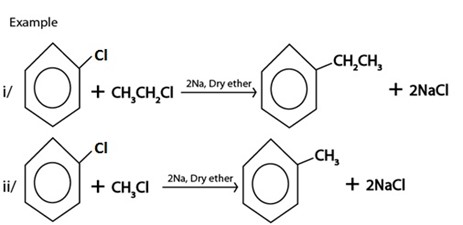
IV. REDUCTION
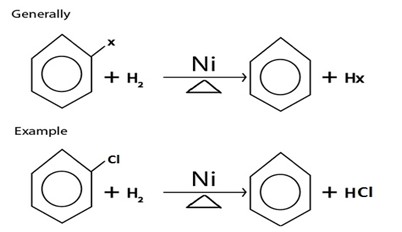
NOTE:
When hydrogen present in excess, cyclohexane if formed.
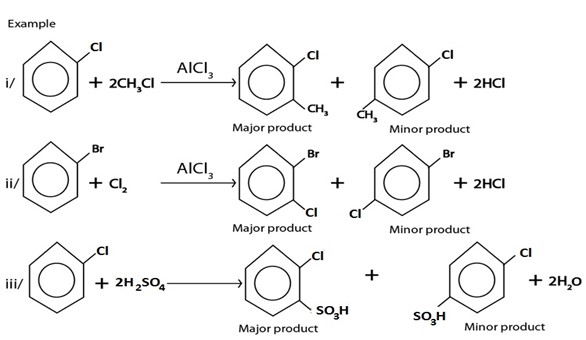
V. ELECTROPHILIC SUBSTITUTION REACTIONS
Under this heading haloarene undergo similar reactions as those of benzene, the only difference is that halogen present in haloarene directs incoming electrophile at ortho and para position forming ortho product as major product.
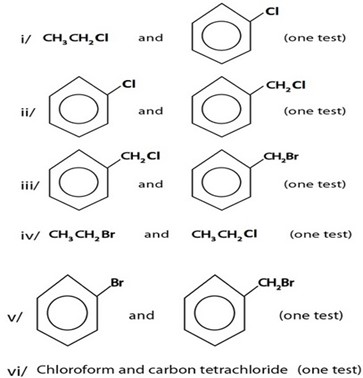
Qn:
Give chemical tests to distinguish each of the following pairs of organic compounds.
ANS.
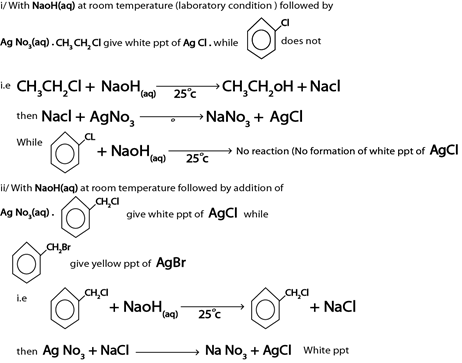
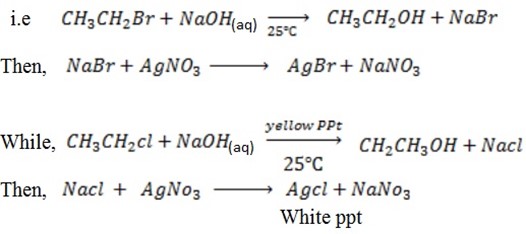
With NaOH(aq) at room temperature followed by addition of 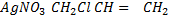 give white ppt of
give white ppt of 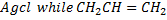 does not.
does not.
i.e
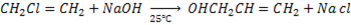
Then,

While
CH3CH=CH2 + NaOH(aq) → No reaction
iv) With NaOH(aq) at room temperature and 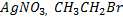 gives yellow ppt of
gives yellow ppt of 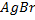 while
while
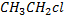 gives white ppt of
gives white ppt of 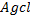
v) With NaOH(aq) at room temperature followed by addition of AgNO3(aq)
Ans
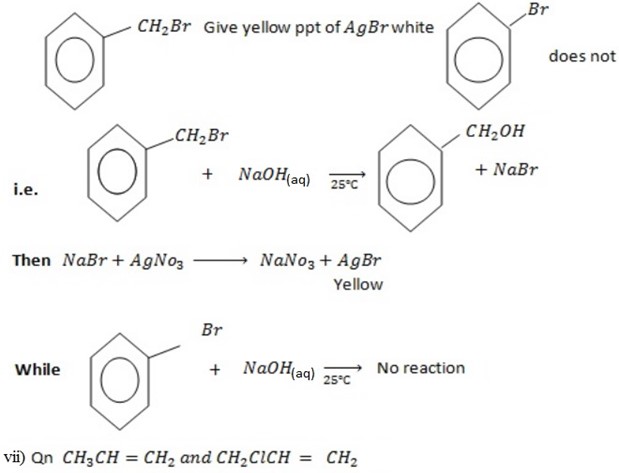
With NaOH(aq) at room temperature followed by addition
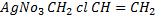 give white ppt of
give white ppt of 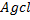 while
while 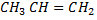 does not
does not
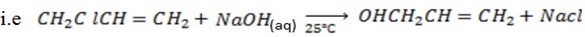
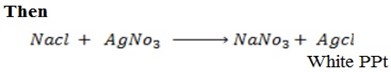
While
CH3CH=CH2 + NaOH(aq) → No reaction
ALCOHOL AND PHENOL
- These are organic compounds with hydroxyl group
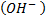 as functional group.
as functional group. - Alcohols and phenol are formed when at least one hydrogen atom of hydrocarbons is replaced by hydroxyl group.
- When the hydrogen atom is replaced from aliphatic hydrocarbon the resulting compound is known as alcohol.
- When the hydrogen atom is replaced from benzene then the resulting compound is known as phenol.
- Most of properties of alcohol resembles with those of phenol due to similarity in their functional group but some properties are different due to difference in their structure.
edu.uptymez.com
CLASSIFICATION OF ALCOHOLS
- Like in haloalkanes, according to number of alkyl groups which are directly bonded to a carbon
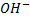 , alcohols can be classified into three categories.
, alcohols can be classified into three categories.
edu.uptymez.com
i. Primary (10) alcohols
ii. Secondary (20) alcohols
iii. Tertiary (30) alcohols
i. PRIMARY (10) ALCOHOLS
- These are alcohols where by a carbon with hydroxyl group is also directly bonded to one alkyl group only.
- Thus far alcohols with one hydroxyl group, the carbon with
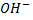 is also directly bonded to two hydrogen atoms.
is also directly bonded to two hydrogen atoms.
edu.uptymez.com
i.e. 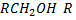 where alkyl group only
where alkyl group only
ii. SECONDARY (20) ALCOHOLS
- These are alcohols where by a carbon with hydroxyl group is also directly bonded to two alkyl groups.
edu.uptymez.com
– Thus for alcohol with one hydroxyl group, the carbon with 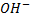 is also directly bonded to one hydrogen atom only.
is also directly bonded to one hydrogen atom only.
i.e 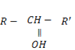
iii. TERTIARY (30 ) ALCOHOL
- These are alcohols where by a carbon with hydroxyl group is also directly bonded to three alkyl groups.
edu.uptymez.com
– Thus there is no hydrogen which is directly bonded to the carbon with 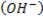 .
.
i.e