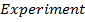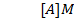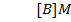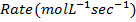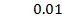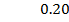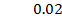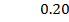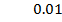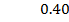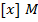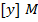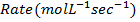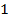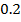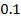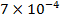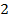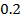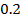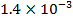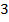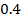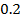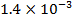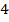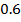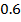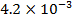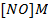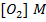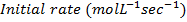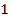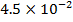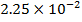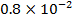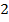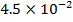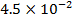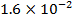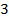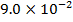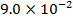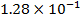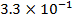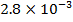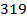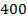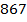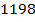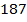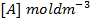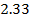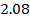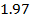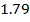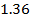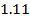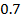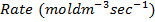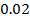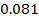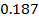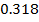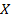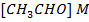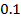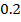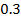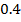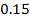Chemical kinetics is the study of speed or rate of chemical reaction under different conditions and mechanisms of the reaction.
The rate of reaction
Rate of reaction is the change in concentration of reactants and products per unit time.
Consider the following hypothetical reaction:

Rate of reaction can be expressed in terms of disappearance of A or B and rate of formation of C or D. But these rate are not the same i.e. B disappears 3 times as fast as A. Therefore rate of disappearance of B is equal to 3 times rate of disappearance of A.
Rate of disappearance of B= 3 times rate of disappearance of A
Similarity;
Rate of formation of C = 2 times rate of disappearance of A
Rate of formation of D = 2 times rate of disappearance of A
Rate of formation of D = 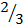 times rate of disappearance of B
times rate of disappearance of B
The rates of disappearance are negative (since concentration decreases with time) and rate of formation are positive (concentration increase with time). But all result in single positive expression for the rate of reaction.
To remove the effect of stoichiometry, the rate for each species is divided by the co-efficient of the molecules in the balanced equation

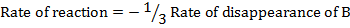
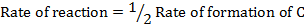
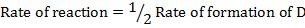
Example: 01
In the above hypothetical reaction, the initial concentration of A is 1.0M and 1minute later was found to be 0.9982M.
i) What is rate of reaction in moles per litre per second?
ii) What is rate of formation of C in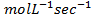 ?
?
Solution;
i) 
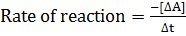
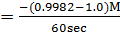
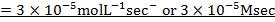
ii) 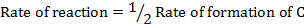

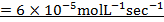
Example: 2
In the reaction; 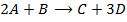
Reactant A is found to be disappearing at a rate of 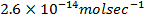
a) What is the rate of reaction?
b) What is the rate of formation of D?
Solution;
a) 
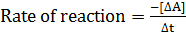
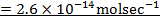
b) 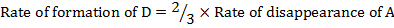
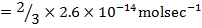
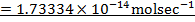
RATE LAW
The law states that “The rate of reaction is direct proportional to the concentration of the reactants each raised to the power of equal to the order of the reaction”

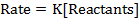
Where K = Rate constant (Velocity constant)
By definition;
Rate constant is a constant of proportionality in the rate equation which is the measure of the speed with which a reaction is taking place at the given temperature
The units of K depends on the order of reaction
Order of reaction this is the number which shows the manner with which the rate of reaction depends on the concentration of reactants.
Order of reaction can only be obtained experimentally and cannot be deduced from overall balanced equation. OR order of reaction is the sum of power of concentration of the reactants in
the rate law or rate equation.
Consider the example of the reaction; 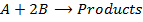
The rate law or rate equation is given by; 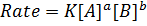
The reaction order with respect to [A] is a and [B] is b and the overall order of reaction is (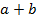 ) and that can be a fraction
) and that can be a fraction
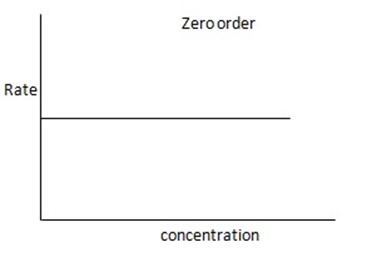
1. Zero order reaction
Is the reaction in which the rate of the reaction is independent on the concentration of the reactants.
Consider the reaction; 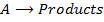
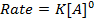
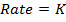
The rate is constant and independent of the concentration of A
Units of rate constant, K
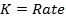
But 

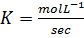
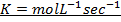
Assignment: Find any two examples of zero order reactions
Graph of rate against concentration A
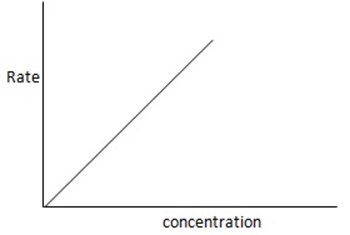
2. First order reaction
Is reaction in which the rate of reaction is directly proportional to the first power of the concentration of the single reaction reactant.
Consider the reaction; 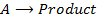
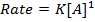
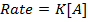
The units of rate constant, K
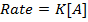
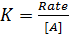
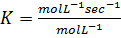
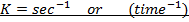
Graph of rate against concentration A
Assignment: Find any 2 reaction of first order reaction
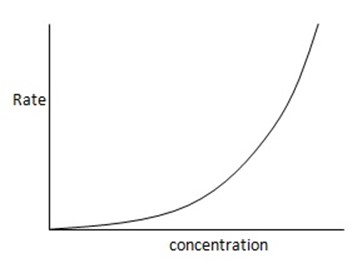
3. Second order reaction
Is the reaction in which rate of reaction is proportional to the second power of concentration of a single reactant or first power of concentration of two reactants (Although rate of reaction
increases with increase in concentration of the reactant, the relationship is not direct)
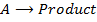 s
s
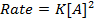
Or 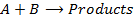
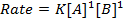
Units of rate constant, K
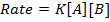
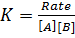
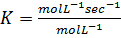
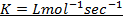
Graph of rate against concentration A
DETERMINATION OF ORDER OF REACTION
This can only be found experimentally.
The order of the reaction can be found by keeping the concentration of one reactant constant and changing the concentration of other at a given temperature.
Example:
1. The following data was obtained for the reaction
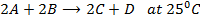
a) What is the order with respect to;
i) Reactant A
ii) Reactant B
iii) Reactant A and B
b) Calculate the velocity constant and the rate of reaction with concentration A and B are 0.01M and 0.1M respectively.
Solution:
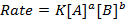
a) i) The value of a is found when the concentration of B must be kept constant so experiment 1 and 2 are considered;
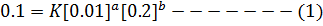
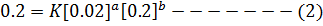
Divide equation (1) by (2)
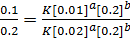
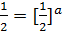
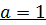
The reaction is first order with respect to reactant A
ii) The value of b is found when the concentration of A must be kept constant, so experiment 1 and 2 are considered;
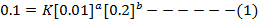
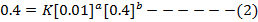
Divide equation (1) by (2)
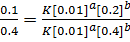
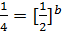
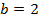
The reaction is second order with respect to reactant B
iii) The overall order of reaction;
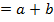
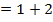
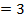
The overall order of the reaction is third order
b) The velocity constant is the average because there are some errors.
Experiment 1:-
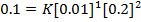
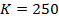
Experiment 2:-
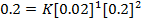
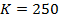
Experiment 3:-
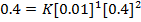
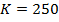
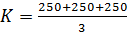
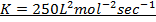
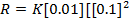
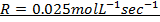
2. The following data were obtained for; 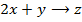
Determine the rate law for the above reaction
Solution:
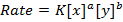
The value of a is found when concentration of y is constant.
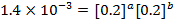
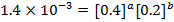
Divide;
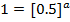
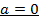
The value of b;
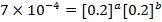
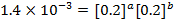
Divide;
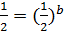
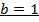
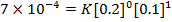
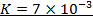
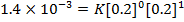
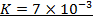
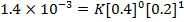
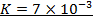
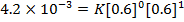
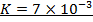
:. 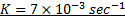
3. The data below was obtained from the reaction;
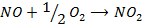 At 298K and 1atm
At 298K and 1atm
Determine:-
i) Rate law
ii) Velocity constant
iii) Rate of reaction in experiment 4
Solution;
i) 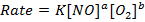
The value of b:-
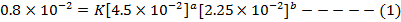
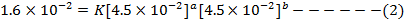
Divide equation 1 by 2
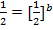
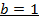
The value of a:-
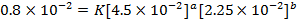
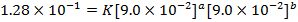
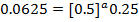
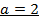
ii) Velocity constant, K
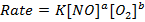
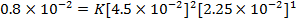
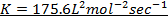
iii) Rate of the reaction in experiment 4
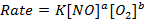
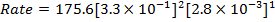
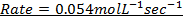
 Note :- If the rate of reaction is not given directly, it can be found from concentration and time. The graph of concentration against time is always a curve for first and second order reaction
Note :- If the rate of reaction is not given directly, it can be found from concentration and time. The graph of concentration against time is always a curve for first and second order reaction
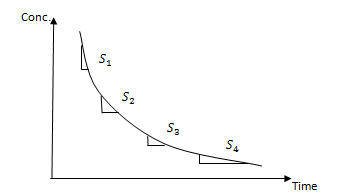
Choose points, draw the tangent and find the slopes
|
slope = Rate of reaction |
edu.uptymez.com
Rate = K[A]m where m= order of reaction
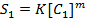 ……………….1
……………….1
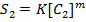 ………………2
………………2
Using these two equations order of reaction can be obtained. For more than two slopes, introduce log on both sides of rate equation
i.e. 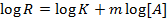
Therefore a graph of log R against log [A] gives a straight line where m = slope (Order of reaction)
Alternatively:-
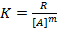
Assume m=1, show 
m=2, show 
m=3, 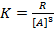
Example:
1. For the reaction 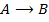 the following data was obtained at 450C
the following data was obtained at 450C
a) Plot a graph of [A] against t:
b) Take tangents to obtain a slope at [A] = 2, 1.167 and 1moldm-3
c) The rate law for the above reaction can be
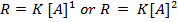
Use of the result in (b) to find which of the rate law actually holds.
2. The following data was obtained from the reaction;
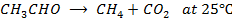
i. Determine the value of n and K in the equation
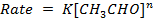
ii. Obtain rate constant and the rate of reaction when concentration of 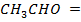
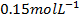
iii. Use the above example to explain the fact that the order of reaction must be determined experimentally and not from the co-efficient of balanced equation
Answers
0.5128 9.4966 * 10-4 = 0.00094966
-0.9676 9.99 *10-4 = 0.00099
4.5099 1.193 * 10-3 = 0.001193=11.93*10-4
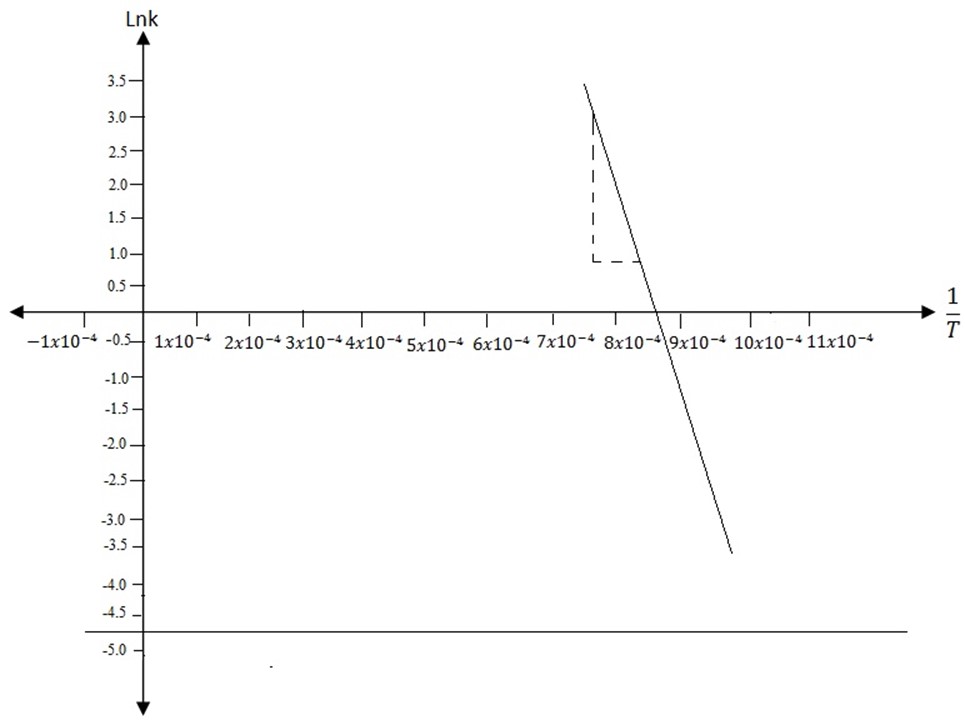
ARRHENIUS EQUATION
From the collision theory, the reaction rate depends the energy and number of collision between molecules and whether the collisions have correct geometry and temperature.
These requirements are summarized by Arrhenius into what is known as the Arrhenius Equation.
K=Ae-EaRT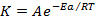
Where; K =Rate /velocity constant.
R= Universal gas constant
A= Frequency factor (per time)
e-EaRT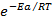 = Fraction of molecules having minimum energy required for the reaction to take place.
= Fraction of molecules having minimum energy required for the reaction to take place.
Frequency factor (A) –Express number of collisions occurring and a fraction of them that has the right geometry.
Application of Arrhenius Equation
i) Determination of Activation Energy
Introduce In on both sides of 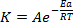
ii) The collision must be energetic enough to break the bond between molecules.
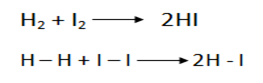
I.e The collision between 2 molecules will not lead to reaction if the kinetic energy cannot provide the necessary activation energy.
As the temperature rises, the number of molecules with energies greater than minimum energy required (ACTIVATION ENERGY) increases.
This factor causes the reaction to be sensitive temperature.