ACTION OF HYDROGEN PEROXIDE
Hydrogen peroxide (H2O2 )
has both oxidizing and reducing power. However its reaction depends on the state of the second reagent whether it has to be reduced or oxidized,
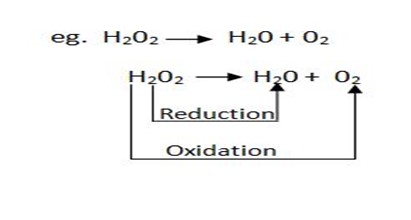
(i) H2O2 as an oxidizing agent
Like any oxidizing agent H2O2 is capable of accepting electrons when treated with any electron donating species and itself being reduced to water.
eg∴
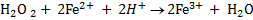
(ii)H202 as reducing agent
H2O2 is capable of supplying electrons when treated with electron accepting species and itself being oxidized to oxygen gas.
E.g.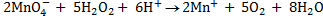
APPLICATION OF REDOX REACTIONS
(i) PERMANGANOMETRY TITRATION
It is applied on permanganometry titration. These are titration in which potassium permanganate is the tit rants and indicators are used. This is because permanganate itself acts as an indicator. It changes its colour due to the formation of manganese (Mn 2+) from manganese (Mn 7+) [in acidic media]
NOTE
Oxidizing power of permanganate depends on the media used.
a) In neutral media or weakly alkali medium.
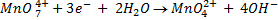
b) In acidic medium
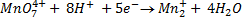
NOTE
Dilute. Sulphuric acid (H2SO4)is only used HCl cannot be used because 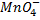 will oxidize it to chlorine gas thus is interfering the power of permanganate
will oxidize it to chlorine gas thus is interfering the power of permanganate
eg. 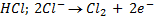
NOTE :– Concentration. 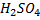 and
and 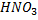 are also not used because they are also oxidizing agents.
are also not used because they are also oxidizing agents.
c) In strong alkali medium.
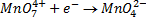
1. 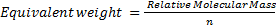
n = number of electrons transferred per mole.
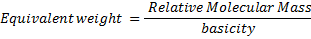
Basicity is the number of H+ per 1 molecule of acid when dissolved in H2O.
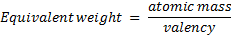
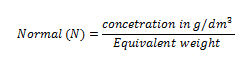


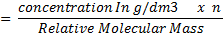
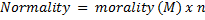

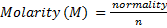
Examples
1.A standardization of 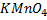 in acidic solution gave the following data:
in acidic solution gave the following data:
0.16g of potassium salt i.e. 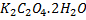 needed
needed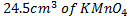 solution. What is the molarity of the solution
solution. What is the molarity of the solution
(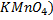
Solution:
(i) To find molarity of K2 C2 04 .2H20
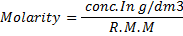
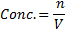
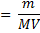

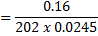
Concentration = 0.032gL-1
202g = 1 mol
0.16g = x
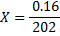
X = 7.92 × 10-4 moles
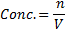
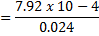
Concentration = 0.032 g dm-3
Molarity= 2.038 x 10-4
(ii) To find molarity of KMno 4 from reaction equation :-
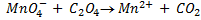
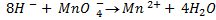
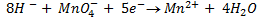
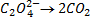
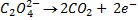
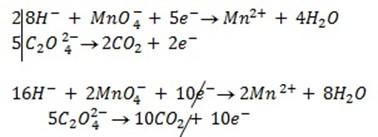
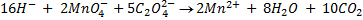
2:5
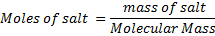
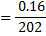
= 7.92 x 10-4 moles
From the reaction equation :-
2 moles of KMnO4 = 5 moles of 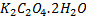
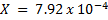
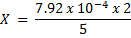
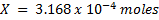
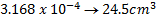
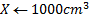
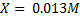
2. Calculate the percentage of iron from the following data in a sample of iron wire:
1.4g of the wire was dissolved in excess dilute sulphuric acid and the solution was made up to 250cm3. 25cm3 of this solution required 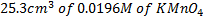 for oxidation. [If it was concentration.
for oxidation. [If it was concentration. 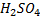 , it could have oxidized Fe to Fe3+ since it is a STRONG oxidizing agent]
, it could have oxidized Fe to Fe3+ since it is a STRONG oxidizing agent]
Solution:
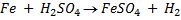 ……………………………………. (1)
……………………………………. (1)
Reaction of 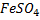 and
and 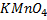
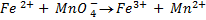

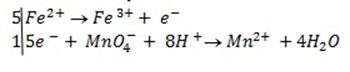
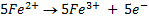
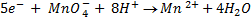
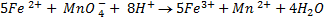
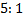
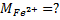
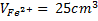
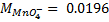
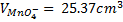
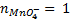
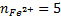
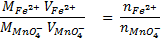
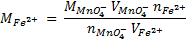
=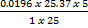
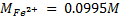
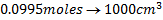
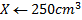
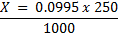
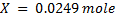 s
s
1 mole of Fe →1 mole of FeSO4
? x mole →0.0249 moles
X = 0.0249 moles of Fe
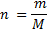
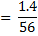
n = 0.025 moles
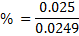
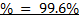
3. 100cm3 of H2O2 solution was diluted to 1 dm3 of solution. 25cm3 of this solution, when acidified with dilute H2SO4 reacted with 47.8cm3 of 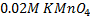 solution. Calculate the concentration of the original H2O2 solution.
solution. Calculate the concentration of the original H2O2 solution.
Solution:
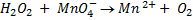
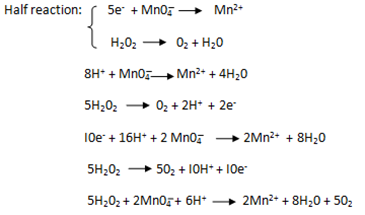
Ratio is 5: 2
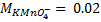
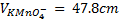
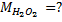
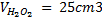
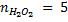
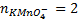
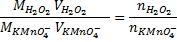
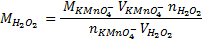
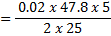
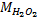 = 0.0956M
= 0.0956M
0.0956 moles → 1000cm3
X → 100cm3
X = 9.5 x 10-3 moles
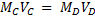
MD = 0.0956
VD = 1000cm3
Mc =?
VC = 100
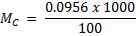
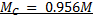
4. 25cm3 of sodium oxalate (Na2C2O4) solution acidified with dilute sulphuric acid and heated to 80°c is titrated with standard KMnO4 solution of concentration 3gl-1. 26.4cm3 of KMnO4 solution was required for complete reaction.
a.Calculate the concentration of oxalate ions in Na2C2O4 in gl-1
b.Hot acidified Na2C2O4 reacts with MnO2 according to the equation.
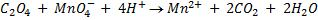
What volume of sodium oxalate (Na2C2O4) will be required to react with 0.1g of MnO2.
Answer
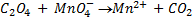
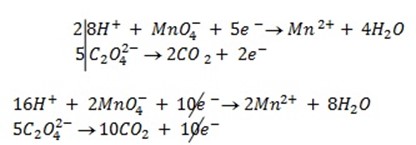
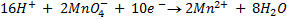
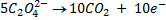
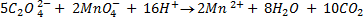
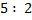
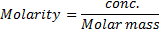
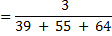
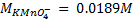
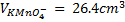
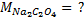
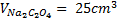
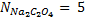
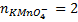
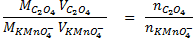
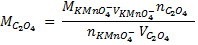
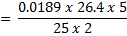
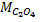 =
=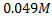
Conc. =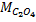 x M.M
x M.M
= 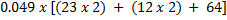
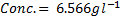
b) 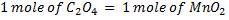
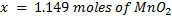
x = 1.149 x 10-3 moles of C2O4
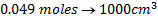
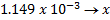
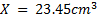
(ii) Iodometry titration
These are titrations in which iodine is produced by using of starch as an indicator. All iodometry
titrations use KI as source of Iodine.
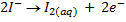
a)If the oxidant is acidified KMnO4
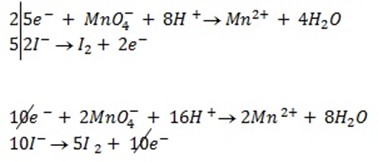
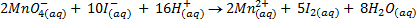 ……………… (1)
……………… (1)
The iodine produced is titrated with 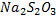 to pale yellow, then add starch and continue titrating until the blue-black colour is discharged.
to pale yellow, then add starch and continue titrating until the blue-black colour is discharged.
Starch is not added at the beginning because the concentration of iodine is large. Iodine react with starch to form the blue black complex hence more volume of 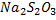 is required to discharge the blue black complex.
is required to discharge the blue black complex.
Reaction with Na2S2O3
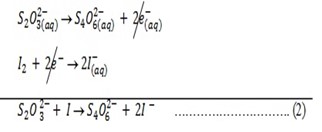
When equation (1) and (2) are combined
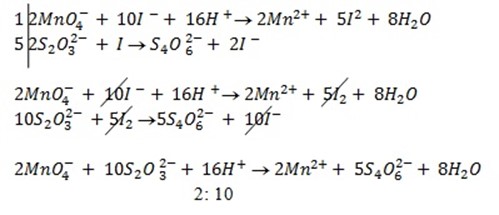
1: 5
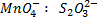
1: 5
b) If the oxidant is acidified K2Cr2O7
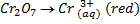
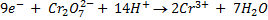
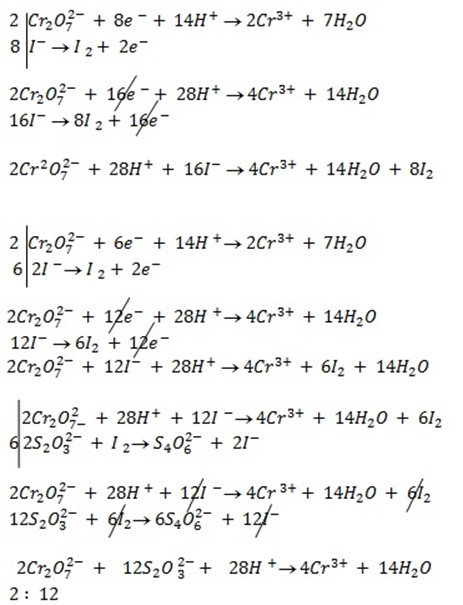
Mole ratio will be
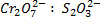
1: 6
c) If the oxidant is acidified KIO3
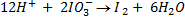
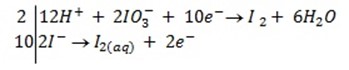
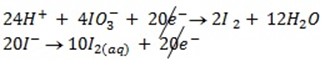
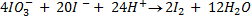
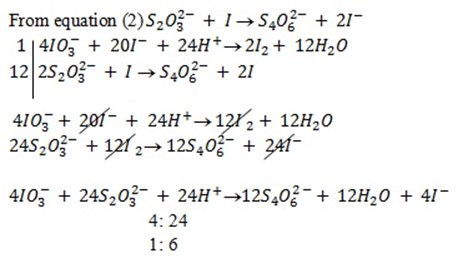
The mole ratio is
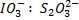
1: 6
ELECTRODE POTENTIAL AND ELECTROCHEMICAL SERIES
ELECTRODE POTENTIAL
Metals have a small tendency to dissolve in solution of their ions producing cations leaving their valency electrons on the metal rod. The metal acquires a negative potential which prevents further release of cations and equilibrium is established
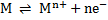 ne–
ne–
⇒ number of electron (s)
As a result the region of solution very close to the rod suffers an increase in charge while the rod carries a layer of negative charge (electrons). Then an electric double layer is set up and this layer is known as “Helmholtz double layer”
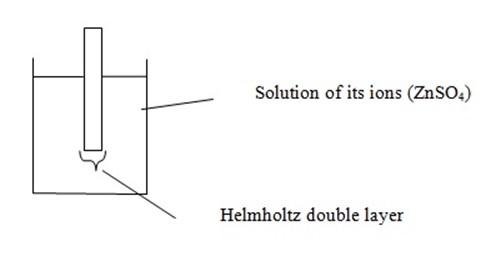
Whenever there is a separation of negative and positive charges we should be able to measure the voltage i.e. voltage between the electrode and surrounding solution and this is called Electrode potential.
Definition:
Electrode potential – is the potential difference formed between an electrode and its hydrated ions.
Magnitude of electrode potential
This depends on the position of equilibrium of reversible reaction. The further to the right the greater is the electron density on the surface of metal and larger is the Potential Difference (P.D) between metal and solution. The opposite is true.
For a given metal the position of equilibrium forward or backward depends on the concentration of solution into which the electrode is dipped
i.e. 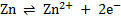
If the concentration of the solution is high the equilibrium will lie towards the left i.e. tendency of zinc rod to dissolve decrease and vice versa.
For different metals placed in solution contain same concentration of their ions at the same temperature i.e. the positions of equilibrium is governed by the overall energy change forming hydrate ions from the metal.
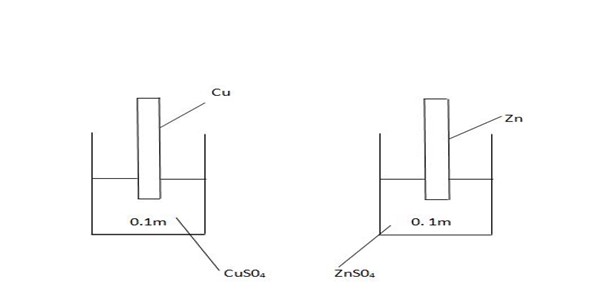
Electrode potential involves the following stages
(i)Atomization of electrode
(ii) Ionization of gaseous atoms
(iii)hydration of ions
Atomization ionization hydration
Zn(s) → Zn (g) → Zn2+ → Zn (aq) 2+
Energy Energy Energy
If the total energy is low, electrode dissolve more easily and its equilibrium will move forward hence large potential (electrode potential) value
Metals with large electrode potential release electrons easily and are good reducing agents