ELECTROCHEMICAL SERIES
Is the series of standard electrode potential with respect to their elements from more negative standard electrode potential to the more positive standard electrode potential.
Is the arrangement of electrodes of elements in order of reducing power
Uses
It is a good guide for predicting reaction that takes place in solution especially displacement reaction.
Displacement reaction
Is a types of reaction in which an atom or element displace another element or atom in a compound
Example
What will happen when magnesium ribbon is added to a solution of AgNo3?
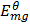 = -2.37v
= -2.37v
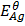 = 0.8v
= 0.8v
The more negative the electrode potential the greater is the reducing power of that element i.e. the more likely it is to give out the electrons and acts as a reducing agent therefore Mg will reduce Ag+ions to Ag (s).
Mg(s) 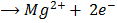
2Ag+(aq)+ 2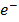
 2Ag(s)
2Ag(s)
Mg (s) + 2Ag+(aq) Mg2+(aq)+ 2Ag (s)
Mg2+(aq)+ 2Ag (s)
Mg(s)/Mg2+(aq)//Ag+(aq)/Ag(s)
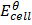 =
= 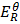 –
– 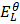
= 0.8 – (-2.37)
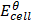 = 3.17v
= 3.17v
Thus, the element higher in electrochemical series will displace the one lower in the series.
2. Displacement of hydrogen from mineral acids
Metals which are higher in the electrochemical series than hydrogen reacts with acids and replaces hydrogen but metals below hydrogen have no action with mineral acids.
Complete the following reaction:-
i. Zn(s) + 2HCl (aq)
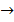 ZnCl2(s) + H2(g)
ZnCl2(s) + H2(g)
ii. Cu(s) + HCl(aq) 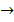 No reaction
No reaction
3. The knowledge of electrochemical series helps as on choosing method for extraction of metals.
Example
Higher most metal can’t be extracted from the oxides by chemical reduction process. This is because they are strong reducing agent hence they can be reduced easily from their oxide by electrolysis.
Questions
For electrolysis, fused or molten metal should be used and not aqueous solution. Why?
Answer:-
In aqueous solution, there are H+ ions. Hence metals prefer to react with element in lower electrochemical series than the metal itself, hence for electrolysis we use fused or molten metal and not aqueous solution.
CORROSION AND ITS PREVENTION
Corrosion is the deterioration of the metals due to the chemical reactions taking place on the surface. Usually, the process is due to the loss of metal to a solution in some forms by a redox reaction (unwanted redox reactions)
For corrosion to occur on the surface of a metal there must be anodic area where a metal can be oxidized to metal ions as electrons are produced.
Anode area:-
M(s) 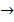 Mn+ + ne+ And cathodes area where electrons are consumed by any of all of several half reactions.
Mn+ + ne+ And cathodes area where electrons are consumed by any of all of several half reactions.
Cathodic reaction:-
2H+(aq) + 2e– 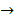 H2 (g)
H2 (g)
2H2 O(l) + 2e–
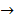 2OH–(aq)+ H 2(g)
2OH–(aq)+ H 2(g)
4e– + O2(g) +H2 O(l)
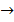 4OH–(aq)
4OH–(aq)
Anodic reactions occur at cracks or around the area with some impurities.
RUSTING OF IRON
The most common corrosion process is rusting of iron
Rust is hydrated iron (III) Oxide (Fe2O3. XH2O) which appears as a reddish brown substance on the surface of the iron bar.
Both water and air (oxygen) are required for rusting to occur.
The presence of dissolved salts and acid in water increases its conductivity and speed up the process of rusting.
If the iron object has free access to oxygen and H2O as in flowing water, a reddish brown ion (III) oxide will be formed which is a rust.
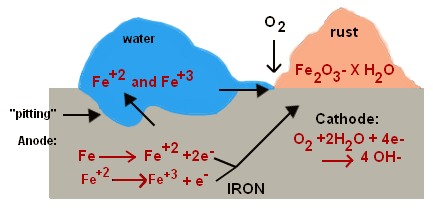
For iron:
Anode: Fe(s)
 Fe2+
Fe2+
(aq)+ 2e–
Cathode:O2(g) + 2H2O(l) + 4e–  4OH–(aq)
4OH–(aq)
2Fe(s) + O2(g) + 2H2 O(l)  2Fe2+(aq)+ 40H–(aq)
2Fe2+(aq)+ 40H–(aq)
(rust)
4Fe(OH)2(s) + O2(g) 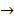 2H2O(l) + 2Fe2 O3.XH2 O
2H2O(l) + 2Fe2 O3.XH2 O
If oxygen is not freely available: The farther oxidation of iron (II) hydroxide is limited to formation of magnetic iron oxide.
6Fe(OH)2(s) + O2(g) 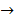 2Fe3 O3.H2O + 4H2O(l)
2Fe3 O3.H2O + 4H2O(l)
Fe3O4 + H2O(l) 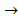 Fe3O4 + 4H2O
Fe3O4 + 4H2O
Black magnetite
Prevention of rusting
Corrosion can’t be made non-spontaneous but it can be prevented by making the rate of the reaction negligible. This can be done by covering the metal surface with protective coating or by providing alternative redox pathways (oxidation-reduction pathways).
Protective coatings are usually of 3 types:
i.Painting: – is the simplest and most common method where a metal surface is properly cleaned and then applied with several layers of rust – proofing paint.
ii.Corrosion inhibitors: – these interfere with flow of charges needed for corrosion to take place i.e. phosphate (II) coating on a surface of iron of steel. Using phosphoric acid serves that purpose.
iii.Galvanization: (sacrificial protection) a metal can also be protected by coating with a thin film of second metal where the second metal is oxidised instead of the 1st metal.
Often iron is coated with another metal like zinc, tin or chromium for protection on the surface.
Note
zinc is preferred to tin because zinc protects iron against rusting when its coating has broken down. This is because it has a more negative reduction potential than iron: it acts as a cathode hence it is not changed. This is called cathodic protection.
Tin protects iron only as long as coating is intact. Once the coating is broken down, tin actually promotes corrosion of iron as iron has more negative reduction potential than tin. Thus iron acts as anode and dissolves while tin acts as cathode and does not change.
Factors which affect corrosion:
i. Position of metal in the electrochemical series(E.C.S). the reactivity of metal depends upon its position in the electrochemical series. More the reactivity, the more likely it is to be corroded.
ii. Presence of impurities in the metal. The impurities help to set up the voltaic cells which increase the speed of corrosion.
iii. Presence of electrolytes: Presences of electrolytes in water also increase the rate of corrosion. E.g. Corrosion of iron in sea water takes place to a large extent than in distilled water.
iv.Presence of CO2 in water: water containing CO2 acts as an electrolyte and increases the flow of electrons from one place to another. (CO2 + H2O) form carbonic acid which dissolves into ions and hence acts as an electrolyte).
Examples
1. Why do you think zinc on iron is sometimes called sacrificial anode?
2. Explain why blocks of Mg can be attached to wills of ship or irons pipes with the aim of preventing rusting.
3. Tin cans are made of Iron coated with thin film of tin. After a crack occurs in the film, a can corrodes much more rapidly than Zinc coated with Iron. Explain this behavior.
4. Why is it that with enough time, corrosion will always defect the protection applied to iron?
ANSWERS
Zinc or Iron is sometimes called sacrificial anode because it after it wears off, the metal can get exposed and hence starts to undergo rust.
Conductivity in solutions
Electrolytes
These are substances which allow electricity to pass through them in their molten state or in form of their aqueous solution, and undergo chemical decomposition e.g., acids, bases and salts.
Classification of electrolytes
All electrolytes do not ionize by the same extent in the solution. According to this we have strong and weak electrolytes
Strong electrolytes are those that ionize completely into ions in the solution
e.g. Salts, mineral acids, some bases.
Weak electrolytes are these that ionize partially into ions in the solution
e.g. in organic acids. HCN, Na4OH.
Electrolytic conduction
When a voltage is applied to the electrodes dipped into an electrolytic solution, ions of the electrolyte move towards their respective electrodes and therefore electric current flows through the electrolytic cell. The process of the electrolyte to conduct electric current is termed as conductance or conductivity.
Like metallic conductors, electrolytic solution also obey ohm’s law which states that “the strength of the current flowing through a conductor is directly proportional to the potential difference applied across the conductor and inversely proportional to the resistance of the conductor
i.e. V = RI.
Resistance of any conductor is directly proportional to the length L and inversely proportional to the area of cross – section.
R 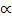
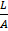
R = ρx 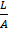
ρ=Resistivity
Resistivity is the resistance of a conductor having unit length and unit area of cross-section. SI unit Ohm-meter (Ὡm).
Conductance is the measure of the ease with which the current flows through a conductor a (λ orΛ)
Conductance is the reciprocal of electric resistance. (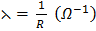 )
)
From the above expression high resistance means low conductance and vice versa.
Conductivity is the reciprocal of resistivity and is also called specific resistance 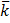 (kappa)
(kappa)
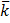 =
=  (
(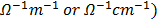
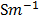
For an electrolytic cell, l is the distance apart between the two electrodes and A is the total area of cross-section of the two electrodes. Therefore, for a given cell, l and A are constant. If the dimensions of the cell are not altered, the ration  is referred as cell constant (K)
is referred as cell constant (K)
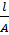 = K
= K
R = ρ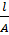
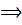
 =
= 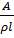
 =
= 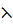 ,
, =
= 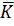
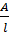 =
= 
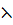 =
= 
Molar conductance (λm)
Is the conductivity of volume of a solution which contains 1 mole of the solute.
Or
It is the conducting power of the ions produced by dissolving 1 mole of an electrolyte in a solution.
Molar conductance is given by 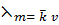 where v = volume containing 1 mole of a solute and is called dilution.
where v = volume containing 1 mole of a solute and is called dilution.
Concentration of an electrolyte depends on the volume i.e.
V =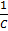
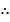
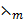 =
= 
Where C is the concentration in mol/dm3 and 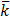 is
is 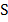 m-1
m-1
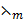 = Ѕm2mol-1 or m2Ὡ -1 mol-1
= Ѕm2mol-1 or m2Ὡ -1 mol-1
Example
1. What is the dilution of 0.2M, NaOH solution?
Solution:
Given l = 0.2mol/dm3
V = 
V = 5dm3mol-1
2.0.0055M silver nitrate has a molar conductivity of 2.98 x 10-3m2 Ω-1 mol-1 . Calculate conductivity of that solution.
Solution
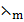 =
= 
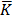 =
= 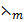 x c
x c
= 2.98 x 10-3 m2Ω-1 mol-1 x 0.0055moldm-3
= 2.98 x 10-3 m2 Ω-1 x 5.5cm-3
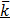 = 0.01529 Ὡ-1 m-1
= 0.01529 Ὡ-1 m-1
3. Calculate the molar conductivity of 0.3M, KOH solution which has a conductivity of 391Ὡ1-1 m-1.
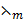 =
= 
= 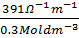
= 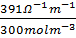
= 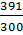
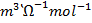
λ∞= 1.303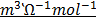
5.The cell constant of the conductivity cell was stated as 0.215 cm-1.
The conductance of the 0.01 moldm-3solution of KNO3was found to be 6.6 x 10-4 Ð…
i. What is the conductivity of the solution?
ii.What results does this give for the molar conductivity of KNO3
Solution:
i) R = ρ
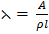 =
= 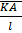 =
= 
K = 0.215cm-1
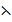 = 6.6 x 10-4 s
= 6.6 x 10-4 s
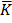 =
= 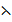 K
K
= 6.6 x 10-4 s x 0.215cm-1
= 1.419 x 10-4 Ð…cm-1
ii) 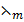 =
= 
= 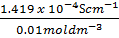
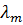 = 14.195
= 14.195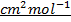
Questions
1. How many grams of acetic acid must be dissolved in 1dm3 of water in order to prepare a solution with a conductivity and molar conductivity of 575cm-1 and 9255cm2mol – 1 respectively?
2. 0.05M NaOH solution offered a resistance of 31.6Ὡ in a conductivity cell at 298K. If the cell constant of a cell is 0.367 cm-1. Calculate the molar conductivity of NaOH solution.
3. The conductivity cell filled with 0.01M KCl has a resistance of 747.5Ὡ at 250c,when the same cell was filled with aqueous solution of 0.05M CaC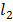 , the resistance was 876Ὡ. Calculate.
, the resistance was 876Ὡ. Calculate.
i.Conductivity of the solution
ii.Molar conductivity of the solution if given 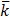 of 0.01M KCl is 0.4114Ð…m-1
of 0.01M KCl is 0.4114Ð…m-1
VARIATION OF MOLAR CONDUCTIVITY WITH CONCENTRATION
The intensity of electricity that can pass through the solution depends on
i.The number of concentration of free ions present in the solution.
ii. Speed with which ions move to their respective electrodes.
An increase in concentration gives an increase in total number of solute particles in a given volume of solution and this might well be expected to give an increased conductivity.
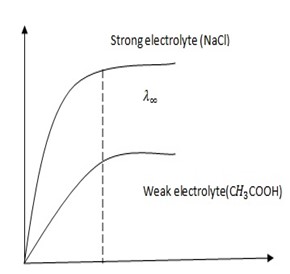
Conduction in strong electrolytes.
The molar conductivity is high since strong electrolytes ionize completely into free ions and the molar conductivity increases slightly in dilution. WHY?
In strong electrolytes, there are vast numbers of ions which are close to each other. These ions tend to interfere with each other as they move towards their respective electrodes. The positive ion are held back by the negative ions and vice versa which in turn interrupts their movement to the electrodes (reduce the speed with which they move)
Dilution ions get separated from each other and at an average distance they can move freely or easily.
NOTE:-
As the dilution increases, there comes a time for amount of interference become small so that further dilution to has no effect.
At this point the molar conductivity remains constant and it is known as molar conductivity at zero concentration 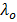 .
.
Conduction in weak electrolyte
The molar conductivity is less because there is less number of particles as the minority of particles are dissociated into ions. On dilution the molar conductivity increases as the molecules dissociate more into ions which increases the number of free ions. Therefore for weak electrolytes the molar conductivity depends on the degree of dissociation of molecules into ions.
Question 1
At infinity dilution, will the molar conductivity of strong and weak electrolyte of same concentration be the same?
Answer
NO, the molar conductivity will be different because it also depends on the size of the ions which would either increase or decrease the speed of ions.
Question 2
Why at infinity dilution, the molar conductivity of weak electrolyte remains constant?
Answer
Because at infinity dilution the molecule must have to dissociate into free ions.