Chemical reactions which takes place in both directions are called reversible reactions .These reactions do not proceed to completion rather to dynamic equilibrium between products and reactants.
As a result of this, the concentration of reactants and products becomes constant (but not equal)
Reactants  products
products
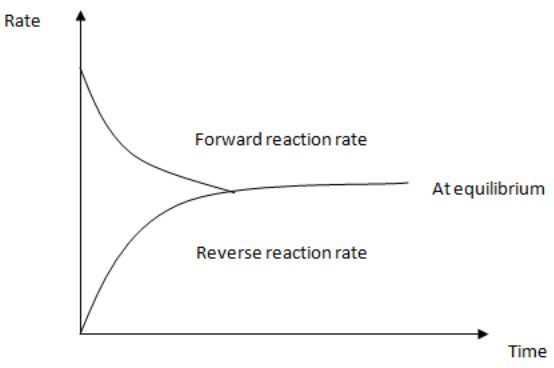
At equilibrium, the rate of forward reaction is equal to the rate of backward reaction.
Note: The concentration of all species in the equilibrium remains constant since both opposing reactions proceed at the same rate.
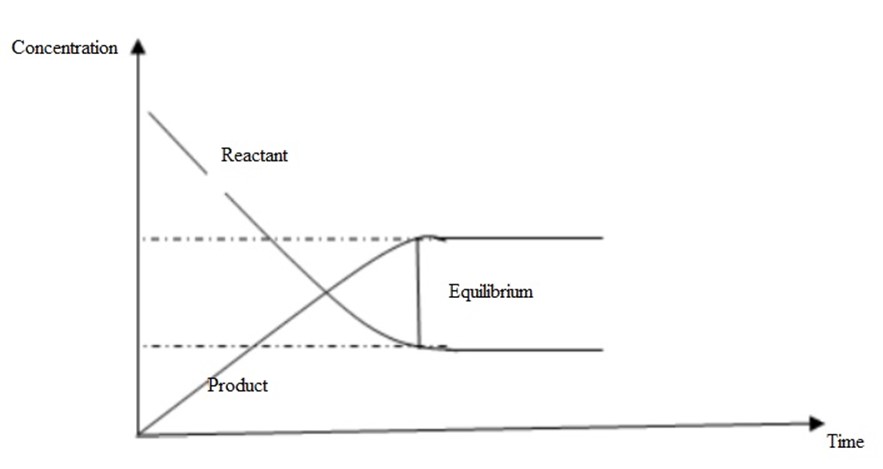
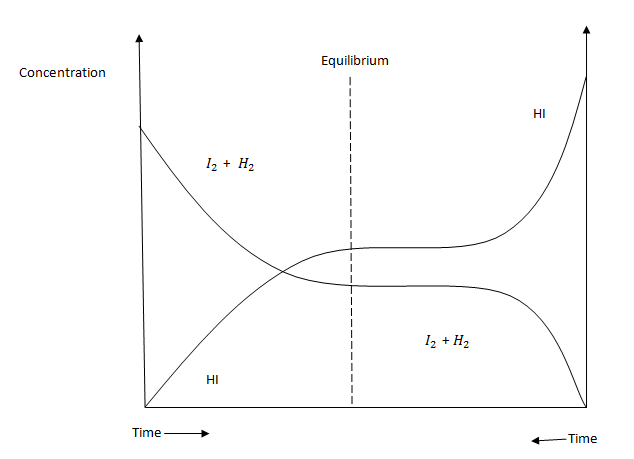
The concentration of reactants decreases with time while those of products increases with time until equilibrium is reached where the
concentrations are constant (not equal).
The equilibrium has been attained with high concentration of products than reactants therefore equilibrium lies on product side.
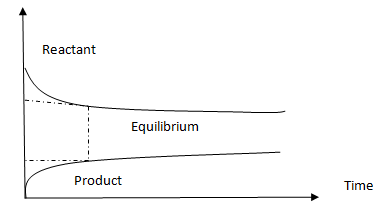
The equilibrium has been attained with high concentration of reactants than products therefore equilibrium lies on reactants side.
The rate of reaction depends on the concentration of reactants. As the concentration of reactants decreases, the rate of forward reaction decreases
too. The rate of backward reaction increases since the concentration of products increases until equilibrium is attained.
Characteristics of chemical equilibrium
1. The equilibrium can be established or attained in a closed system (no part of the reactants or products is allowed to escape out.)
2. The equilibrium can be initiated from either side .The state of equilibrium of a reversible reaction can be approached whether we start from
reactants or products.
Consider the reactions:-
H2(g) + 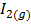
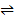 2HI(g)
2HI(g)
3. Constancy of concentration
When a chemical equilibrium is attained, concentration of various species in the reaction mixture becomes constant.
4. A catalyst cannot change the equilibrium point. When a catalyst is added to a system in equilibrium, it speeds up the rate of both forward and
backward reactions to equal extent.Therefore a catalyst cannot change equilibrium point except that it is reached earlier.
Types of chemical equilibrium
1. Homogeneous equilibrium:
This is equilibrium where reactant and products are in the same physical states i.e. all solids, all liquids or all gases.
E.g. H2 (g) + I2 (g) 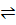 2HI (g)
2HI (g)
N2 (g) + 3H 2(g) 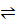 2NH3 (g)
2NH3 (g)
2. Heterogeneous equilibrium;
This is equilibrium when the reactants and product are in the different physical states
CaCO3(s) 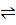 CaO(s) + CO2 (g)
CaO(s) + CO2 (g)
3Fe (S) + 4H2O (g) 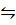 Fe3O4(s) + 4H2 (g)
Fe3O4(s) + 4H2 (g)
3. Ionic equilibrium:
This is an equilibrium which involves ions.
E.g.
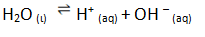
LAW OF MASS ACTION
The law relates the rates of reactions and the concentration of the reacting substances. The law states that, “the rate of a chemical reaction is directly proportional to the product of the molar concentration of the reactants at constant temperature, at any given time”.
The molar concentration means number of moles per litre and is also called active mass
Consider the following simple reactions:
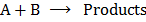
The rate of the reaction, R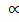
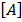
R = K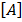
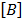 Rate equation
Rate equation
Or R = K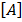
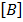
Where [A] and [B] are the molar concentrations of the reactant A and B respectively and that K is a constant of proportionality known as rate constant or velocity constant.
If the concentration of each of the reactants involved in the reaction is unity i.e. the concentration of [A] = [B] = 1, thus the rate constant of a
reaction at a given temperature may be defined as a rate of the reaction when the concentration of each of the reactants is unity.
Generally for a reaction
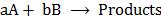
Where a and b are stoichiometric coefficients or mole ratio of the reactants, A and B
r = K [A]a [B]b or R=K [A]a [B]b
LAW OF CHEMICAL EQUILIBRIUM AND EQUILIBRIUM CONSTANT
The law of mass action is applied to reversible reaction to derive a mathematical expression for equilibrium constant known as the law of chemical equilibrium.
Consider a simple chemical reaction (reversible)
A+B 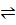 C+D
C+D
The forward reaction is A+B 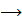 C+D
C+D
Rate of forward (Rf) 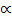 [A] [B]
[A] [B]
Rf = Kf [A] [B]
Kf = Rate constant for forward reaction
Similarly for the backward reaction
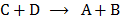
Rate of backward (Rb) 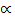 [C] [D]
[C] [D]
Rb = Kb [C] [D]
Kb = Rate constant for backward reaction
At equilibrium both rates are equal
Rforward = Rbackward
Kf [A] [B] = Kb[C] [D]
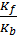 =
= 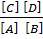
Ke = 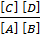
Generally:-
For the reaction
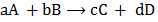
The equilibrium constant for this expression is given by
K e = 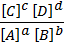
K e is changed to K c for equilibrium concentration (equilibrium constant) .
K c = 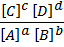
Where K c is equilibrium concentration (equilibrium constant)
[C], [D] etc are molar concentration of species in mol per litre a, b etc are mole ratios or stoichiometric coefficients.
But for gaseous equilibrium we know that PV= nRT
PV = nRT
 =
= 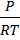
Concentration of a gas is directly proportional to partial pressure therefore the equilibrium constant can be represented in terms of both concentration and partial pressure.
For the above equilibrium;
K p = 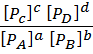
Example1.
1.N2 O4 (g) dissociates to give NO2 (g)
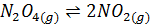
K c = 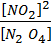
K p = 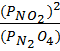
2. H2 (g) + I2 (g) 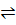 2HI (g)
2HI (g)
K c = 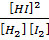
K p =
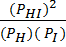
The law of chemical equilibrium states that, “For any system in equilibrium at a given temperature, the ratio of product of concentration of products to the product of the concentration of reactants raised to the power of their mole ratios is constant”.