UNITS OF EQUILIBRIUM CONSTANT
The units of equilibrium constants, K c and K p depends on the number of moles of reactants and products involved in the reaction
1. N2 (g) + O2 (g) 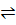 2NO (g)
2NO (g)
K p = 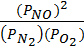
K p = 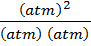 = Unit less
= Unit less
2. N2 (g) + 3H2(g) 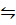 2NH3 (g)
2NH3 (g)
K p = 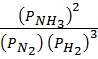
K p =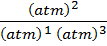
K p = 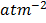
K c = 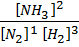
= 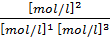
K c = 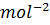
A large value of K p or K c means the equilibrium lies on the sides of the product and a small value of K c and K p means equilibrium lies on the sides of the reactants thus the equilibrium constant shows to what extent the reactant are converted to the product.
If K c is greater than 103, products pre- dominate over reactants equilibrium therefore the reaction proceeds nearly to completion.
If K c is less than 10-3, reactants dominate over products, the reaction proceeds to very small extent.
If K c is in the range of 10-3 and 103, appreciable concentrations of both reactants and products are present.
SOLIDS AND PURE LIQUIDS IN EQUILIBRIUM EXPRESSION
The concentration of a solid or pure liquid (but not a solution) is proportional to its density. Therefore, their densities are not affected by any gas pressure hence remain constant; hence their concentration never appear in the equilibrium expression.
Example
1. What is the equilibrium expression for the following reactions?
i) 3Fe (g) + 4H2O (g) 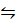 Fe3O4 + 4H2 (g)
Fe3O4 + 4H2 (g)
Solution
K c =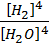 OR K p =
OR K p = 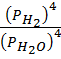
ii) PCl5
(g) 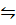 PCl3 (g) + Cl 2
PCl3 (g) + Cl 2
(g)
K c = 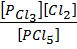
K p = 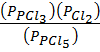
iii) HCl (g) + Li H(S) 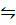 H2 (g) +LiCl(s)
H2 (g) +LiCl(s)
K c = 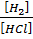
K p = 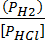
iv) Cu (OH) 2(s) 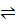 Cu2+ (aq) + 2OH –(aq)
Cu2+ (aq) + 2OH –(aq)
K c = [Cu 2+] [OH –] 2
v) CaCO3(s) 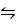 CaO(s) + CO 2(g)
CaO(s) + CO 2(g)
K c = [CO2]
vi) [Cu(NH3)4]2+(aq) 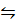 Cu2+(aq) + 4NH3(aq)
Cu2+(aq) + 4NH3(aq)
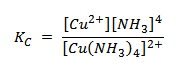
2. The equilibrium constant for the synthesis of HCl, HBr and HI are given below;
H2 (g) +Cl2 (g) 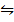 2HCl (g) KC =1017
2HCl (g) KC =1017
H2 (g) + Br2 (g) 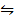 2HBr (g) KC=109
2HBr (g) KC=109
H2 (g) +I 2(g) 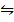 2HI (g) KC=10
2HI (g) KC=10
3. a) What do the value of Kc tell you about the extent of each reaction?
b) Which of these reactions would you regard as complete conversion? Why?
Answers
a) For all the 3 reactions, the equilibrium lies on the product side (i.e. RHS) and the extent of formation of HCl is greater than HBr which in turn is greater than HI.
b) For the 1st and 2nd reaction, it can be regarded as complete conversion because products pre-dominates over reactants at equilibrium.
Characteristics of equilibrium constant
1. Equilibrium constant is applicable only when the concentrations of reactants and products have attained their equilibrium.
2. The value of equilibrium concentration is independent of the original concentration of reactants.
3. The equilibrium constant has a definite value for every reaction at a particular temperature.
4. For a reversible reaction the equilibrium constant for the forwarded reaction is the inverse of the equilibrium constant for the backward reaction.
5. The value of equilibrium constant tells the extent to which reaction proceeds in the forward or reverse direction.
6. Equilibrium constant is independent of the presence of catalyst. This is because the catalyst affects the rate of forward and backward reaction equally.
Relationship between K c and K p for a gaseous equilibrium
For any given reaction, Kc or Kp is a function of the reaction itself and temperature.
Consider the following gaseous equilibrium
aA (g) + bB(g) 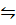 cC (g) + dD (g)
cC (g) + dD (g)
K c = 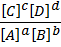 —————— (1)
—————— (1)
K p = 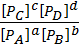 ————– (2)
————– (2)
From PV= nRT
[X] =  =
= 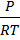
I.e. [A] = 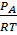 , [B] =
, [B] = 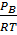
Substitute these concentration in terms of partial pressures in equation (1)
K c = 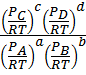
= 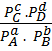 .
. 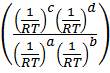
= K p 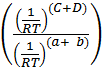
= K p 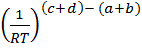
But; (c+d) = total number of moles in the products.
(a +b) = total number of moles in the reactants.
(c +d) – (a + b) = The difference between moles of products and reactants
So, (c +d)-(a + b) = Δn
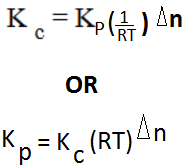
Thus, the numerical value of K p and K c are equal when there is the same number of moles on products and reactants side numerically.
Example:
1. What is the relationship between K p and K c in the following reactions?
2H2 (g) + O2 (g)  2H2 O (g)
2H2 O (g)
From; K p = K c (RT) Δn
= K c (RT) (2- (1+2))
K p = Kc (RT)-1
ii) Derive the relationship between K p and k c for the particular reaction.
2H2 (g) + O2 (g)  2H 2O (g)
2H 2O (g)
K c = 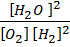 …………… (1)
…………… (1)
K p = 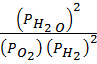 ……….. (2)
……….. (2)
From PV = nRT
P =  RT
RT
P = [X] RT
 = [H 2] RT ,
= [H 2] RT , 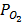 = [
= [ 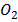 ] RT………etc
] RT………etc
Substitute the partial pressure in equation (2)
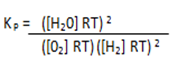
K p =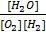 .
. 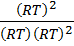
K p = K c (RT) (2-(1+2))
K p = K c (RT)-1
2. a) Derive the relationship between K p and K c for ammonic synthesis.
N2 (g) + 3H2 (g) 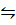 2NH3 (g)
2NH3 (g)
K c = 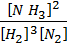 ………… (1)
………… (1)
K P = 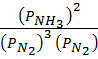 …….. (2)
…….. (2)
From PV = nRT
P =  RT
RT
P = [X] RT
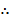
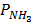 = [
= [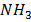 ] RT,
] RT, 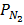 = [
= [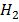 ] RT,
] RT, 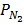 = [
= [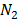 ] RT
] RT
Substitute the partial pressure in equation (2)
K p = 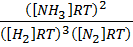
K P =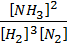
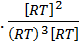
K p = KC x 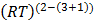
K p = K C.(RT)-2
b) If Kc = 0.105 mol-2dm6 at 4720C. Calculate K p
[R=8.31 dm3 KPa mol-1 K-1]
Solution:
K p = K c x (RT)-2
= 0.105 x (8.31x 745)-2
= 0.105 x (6190.95)-2
= 2.7395x 10-9(KPa)-2
DETERMINATION OF EQUILIBRIUM CONSTANT
Consider the reaction:-
CH3CH2OH + CH3COOH  CH 3COOCH2 CH3 + H2O
CH 3COOCH2 CH3 + H2O
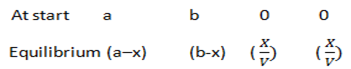
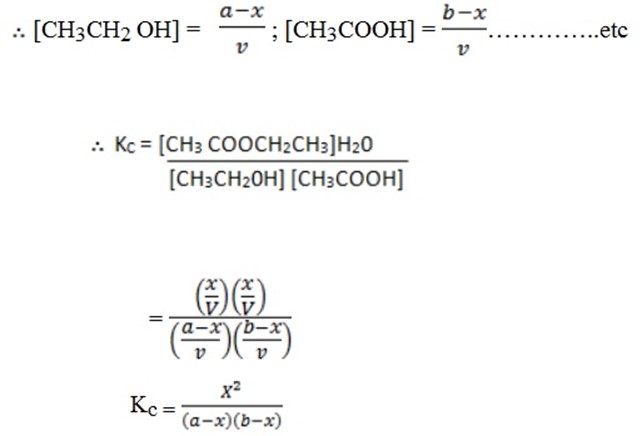
Example:
1. An equilibrium system for the reaction between H2 and I2, to form HI at 670K in 5l flask contains 0.4 moles of H2, 0.4 moles of I2 and 2.4 moles of HI. Calculate the equilibrium constant K c.
H2 (g) +I2 (g) 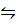 2HI (g)
2HI (g)
K c = 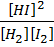
[HI] = 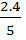 = 0.48
= 0.48 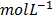
[H2] = 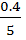 = 0.08
= 0.08 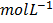
[I2] =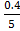 = 0.08
= 0.08 
 K c =
K c = 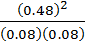
K c = 36
2. A mixture of 1.0 x10-3 moldm-3 H 2 and 2.0 x 10-3 moldm-3 I2 are placed into a container at 4500C. After equilibrium was reached the HI concentration was found to be 1.87 x 10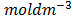 . Calculate the equilibrium constant.
. Calculate the equilibrium constant.
H2 (g) + I2 (g) 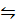 2HI (g)
2HI (g)
At t=0 a b 0
Equilibrium: a-x b-x 2x
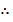 2x = 1.87 x 10-3
2x = 1.87 x 10-3
X= 9.35 x 10-4moldm -3
a=1.0 x 10-3
a-x = 1.0 x 10-3 – 9.35x 10-4
= 6.5 x 10-5moldm-3
b = 2.0 x 10-3
b -x=2.0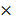 10-3– 9.35
10-3– 9.35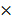 10-4
10-4
=1.065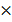 10-3moldm-3
10-3moldm-3
K c = 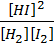
= 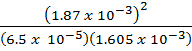
K c = 50.51
3. a) It was found that if 1 mole of acetic acid and half a mole of ethanol react to equilibrium at certain temperature, 0.422 moles of ethyl acetate are produced. Show that the equilibrium constant for this reaction is about 4.
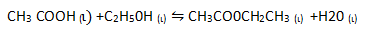
Start: – 1mol 0.5mol 0 0
Equilibrium: 1-0.422 0.5-0.422 0.422 x
0.578 0.078 0.422 0.422
Equilibrium 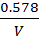
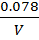
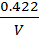
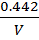
Concentration
K c = 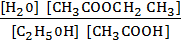
= 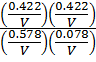
K c = 3.95
K c ≈ 4 shown
b) From the same reaction above, 3moles of acetic acid and 5 moles of ethanol reacted. Find the amounts which will be present equilibrium.
CH3COOH (l) + C2H5OH (l) 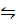 CH3COOCH2CH3 (I) + H2O (I)
CH3COOCH2CH3 (I) + H2O (I)
t= 0 3 5 0 0
t = t (3-x) (5-x) x x
Equilibrium
Concentration 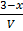
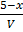
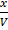
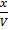
K c = 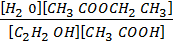
4 = 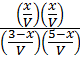
4 = 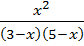
4 = 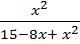
X2 = 60 – 32x + 4x2
3x2 – 32x + 60 = 0
X = 8.24 or x = 2.43
Logically; x = 2.43
At equilibrium CH3COOH 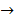 3 – 2.43 = 0.57 moles
3 – 2.43 = 0.57 moles
C2 H5 OH 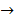 5 – 2.43 = 2.57 moles
5 – 2.43 = 2.57 moles
4 .For the reaction;
CO2 (g) + H2 (g) 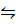 CO (g) + H2 O (g)
CO (g) + H2 O (g)
The value of K c at 5520C is 0.137. If 5moles of CO 2, 5moles of H2, 1 mole of CO and 1 mole of H2 O are initially present, what is the actual concentration of CO2, H2, CO and H2O at equilibrium?
CO2 (g) + H2 (g) 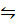 CO2 (g) + H2 O (g)
CO2 (g) + H2 O (g)
At start; 5 1 1 1
At equilibrium 5-x 1-x 1x 1x
K c = 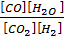
0.137 = 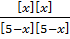
0.137 = 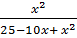
3.425 – 1.37x + 0.137x2 = X2
3.425 – 1.37x + 0.137 x2–x2 =0
3.425 -1.37x – 0.863x2 =0
x =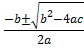
x = 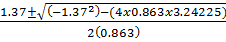
x =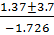 = x =1.349
= x =1.349
COMBINING EQUILIBRIUM REACTIONS
1. Reversing an equilibrium reaction
Consider the reaction
PCl5 (g) 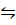 PCl3 (g) +Cl2 (g)………………. (i)
PCl3 (g) +Cl2 (g)………………. (i)
K c (i) =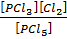 …………………….. (i)
…………………….. (i)
Reversing the reaction;
PCl3 (g) + Cl2 (g) 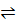 PCl5 (g)
PCl5 (g)
K c (ii) = 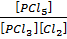 ………………. (2)
………………. (2)
When reaction equation is reversed, the equilibrium constant is reciprocated i.e. from equation (1) and (2)
K c(ii) =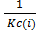
2. Multiplying an equilibrium reaction by a number
When the stoichiometric coefficient of a balanced equation is multiplied by the same factor, the equilibrium constant for the new equation is old equilibrium constant raised to the power of the multiplied factor.
PCl5 (g) 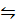 PCl3 (g) + Cl (g)
PCl3 (g) + Cl (g)
K c (i) = 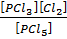
If the equation (i) is multiplied by ½
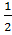 PCl5 (g)
PCl5 (g) 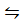
 PCl3 (g) +
PCl3 (g) +  Cl2 (g)
Cl2 (g)
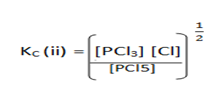

Note; if an equilibrium reaction is multiplied by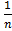 then;
then;
K c(ii ) = 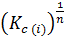
K c(ii) =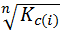
Example: The K c for the reaction below is 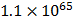
S (s) +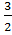 O2 (g)
O2 (g) 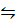 SO3 (g); what is the K c for SO3 Equilibriate with S + O2
SO3 (g); what is the K c for SO3 Equilibriate with S + O2
2 SO3 (g) 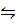 2S(s) + 302(g)
2S(s) + 302(g)
S (s) +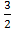 O2 (g)
O2 (g) 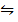 SO3 (g)………………. (I)
SO3 (g)………………. (I)
2SO3 (g) 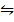 2S(s) + 302(g)…………………. (2)
2S(s) + 302(g)…………………. (2)
Equation (2) has been reversed and multiplied by 2
When reversed
K c (i) = 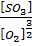 = 1.1×1065
= 1.1×1065
K c (ii) = 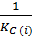
K c (ii) = 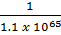
K c (ii) = 9.09 x 10-66
When multiplied
K c (ii) = 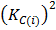
3. Adding the equilibrium.
The equilibrium constant for the reaction
i) 2HCl(g) 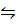 Cl2(g) +H2(g)
Cl2(g) +H2(g)
K c (i) = 4.17x 10-34 (At 25 0 c)
The equilibrium constant for reaction
ii) I2 (g) + Cl2 (g) 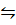 2ICl (g)
2ICl (g)
K c (ii) = 2.1 x 105 (At 25 0c)
Calculate the equilibrium constant for the reaction
iii) 2HCl (g) + I2 (g) 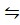 2ICl (g) + H2 (g)
2ICl (g) + H2 (g)
K c (iii) =?
K c (i) = 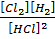
K c (ii) = 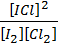
K c (iii) = 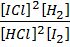
When equation (i) + equation (ii) = equation (iii), Hence
K c (iii) = K c (ii) x K c (i)
= (2.1 x 105) x (4.17 x 10-34)
K c (iii) = 8.757 x 10-29
(mol dm-3)1/2
Question 1:
The following are reactions which occurs at 3500K
i) 2H2 (g) +O2 (g) 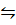 2H2O (g) K p (i) = 26.4 atm-1
2H2O (g) K p (i) = 26.4 atm-1
ii) 2CO (g) +O2 (g) 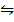 2CO2 (g) K p (ii) = 0.376 atm-1
2CO2 (g) K p (ii) = 0.376 atm-1
Calculate equilibrium constant for reaction
CO 2 (g) + H 2 (g) 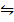 CO (g) + H2O (g)
CO (g) + H2O (g)
K p (iii) =?
Question 2
.Determine the Equilibrium constant for reaction;
 M2(g) +
M2(g) +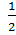 O2 (g) +
O2 (g) +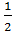 Br2 (g)
Br2 (g) 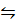 NOBr (g)
NOBr (g)
Given that:-
i/ 2NO (g) 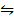 N2(g) + O2(g) K c = 2.4 x
N2(g) + O2(g) K c = 2.4 x 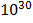
ii/ NO (g) +  Br2
Br2
(g)  NOBr K c = 1.4
NOBr K c = 1.4
Answers
i) 2H2 (g) + O2
(g)  2H2 O (g) K p (i) =26.4atm-1
2H2 O (g) K p (i) =26.4atm-1
ii) 2CO (g) + O2 (g)  2CO2
2CO2
(g) K p (ii) =0.376 atm-1
iii) CO2 (g) + H2 (g)  CO (g) + H2O (g) K p (iii) =?
CO (g) + H2O (g) K p (iii) =?
K p (i) = 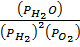
K p (ii) = 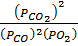
K p (iii) = 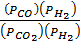
Reverse (ii), multiply (i) and (ii) by ½
i)  (2H2 (g) +O2 (g)
(2H2 (g) +O2 (g) 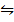 H2O (g)) K p (i) =
H2O (g)) K p (i) = 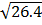
i) H2 (g) + 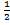 O 2
O 2 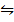 H2O (g) K p(i) = 5.138 atm-1
H2O (g) K p(i) = 5.138 atm-1
Reverse (ii)
ii) 2CO2 (g) 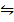 2CO (g) +O2 (g) K p (ii) =
2CO (g) +O2 (g) K p (ii) = 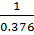
= 2.6595 atm-1
Now multiply by (ii) by ½
 (2CO2 (g)
(2CO2 (g) 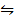 2CO (g) +02(g)) K p (ii) =
2CO (g) +02(g)) K p (ii) =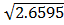
ii) CO2 (g) 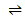 CO (g) +
CO (g) +  O2 (g) =1.63079
O2 (g) =1.63079
Add equation (i) + (ii), then
K p (iii) = K p (i) x K p (ii)
= 5.138 x 1.63079
=8.379 atm-1
2. i) 2NO(g) 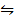 N2(g) + O2(g) K c(i) = 2.4 x 1030
N2(g) + O2(g) K c(i) = 2.4 x 1030
ii) NO (g) + 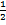 Br (g)
Br (g) 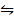 NOBr (g) K c (ii) =14
NOBr (g) K c (ii) =14
iii) 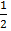 N2 (g) +
N2 (g) +  O2 (g) +
O2 (g) +  Br (g)
Br (g) 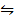 NOBr(g) K c (iii) =?
NOBr(g) K c (iii) =?
Reverse ……. (i)
O2 (g) + N2 (g) 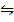 2NO (g) K c (i) =
2NO (g) K c (i) = 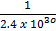
=4.167 x 10-31
Now multiply (i) by ½
 O2 (g) +
O2 (g) +  N2 (g)
N2 (g) 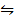 NO (g) K c (i) =
NO (g) K c (i) =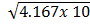 -31
-31
=6.455 x 10-16
Equation (i) + (ii) = (iii)
Therefore:
K c (iii) = K
c (i) x K c (ii)
= 6.455 x 10-16 x 1.4
= 9.0369 x 10-16(mol dm-3)1/2
3. The equilibrium constants for the reactions which have been determined at 878K are as follows:-
i) COO (s) + H2 (g) 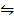 CO(s) + H2O (g) K1 = 67
CO(s) + H2O (g) K1 = 67
ii) COO(s) + CO (g) 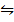 CO(s) + CO2 (g) K2 = 490
CO(s) + CO2 (g) K2 = 490
Using these information, calculate K’s (at the same temperature) for;
iii) CO2 (g) +H2 (g) 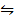 CO2 (g) + H2O (g) K3 =?
CO2 (g) + H2O (g) K3 =?
And commercially important water gas reaction
iv) CO (g) + H2O(g) 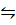 CO2(g)+ H2(g) K4=?
CO2(g)+ H2(g) K4=?
Reverse (ii)
(ii)CO2 (g) + CO(s) 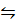 CO (g) + COO (g) K2=2.0408 x 10-3
CO (g) + COO (g) K2=2.0408 x 10-3
Equation (i) + (ii) = (iii)
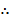 K3 = K 1 x K2
K3 = K 1 x K2
= 67 x 2.0408 x 10-3
K3 = 0.1367
To find K4
i) COO (s) +H 2 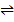 CO (s) + H2O (g) K1= 67
CO (s) + H2O (g) K1= 67
ii) COO(s) + CO (g) 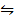 CO (s) + CO2 (g) K2 = 490
CO (s) + CO2 (g) K2 = 490
iii) CO2 (g) + H2 (g) 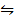 CO (g) + H2O (g) K3 = 0.1367
CO (g) + H2O (g) K3 = 0.1367
iv) CO (g) + H2O (g) 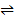 CO2 (g) + H2 (g) K4 =?
CO2 (g) + H2 (g) K4 =?
When equation (iii) is reversed, it is equal to equation (iv)
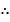 K4 =
K4 = 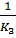
= 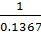
= 7.315
4. The heterogeneous equilibrium
i) Fe(s) + H2O 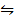 FeO(s) + H2 (g)
FeO(s) + H2 (g)
ii) Fe(s) + CO2 (g) 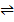 FeO(s) + CO (g)
FeO(s) + CO (g)
Have been studied at 800 0c and 1000 0c.Also the rate (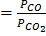 ) is constant = 1.81 at 8000c and 2.48 at 1000 0c.
) is constant = 1.81 at 8000c and 2.48 at 1000 0c.
i) Why are the ratios constant?
ii) Calculate equilibrium constant at two temperatures of the reaction
iii) H2O (g) + CO (g) 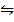 H2 (g) + CO2 (g)
H2 (g) + CO2 (g)
Answer:
i) The ratios are constant because for any system in equilibrium at a given temperature, the ratio of products of concentration of products to the product of concentration of reactants raised to the point of their mole ratios is constant.
ii) At 800 0C
K p1 =2
K p2 =1.81
K p3 =?
(i) Fe(s) + H2O(l) 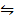 Fe(s) + H2 (g) K p (i) =2
Fe(s) + H2 (g) K p (i) =2
Reversed (ii) CO (g) + FeO(s) 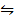 Fe(s) + CO2 (g) K p (ii) =?
Fe(s) + CO2 (g) K p (ii) =?
Equation (i) + (ii) = (iii)
Kp1 x Kp2=Kp3
Kp3 = Kp1 x Kp2
=2 x 0.55
Kp3 = 1.1
At 1000 0c
Kp1 = 1.49
K p2 =2.48
i) Fe(s) + H2O (g) 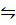 FeO(s) + H2 (g) K p1 =1.49
FeO(s) + H2 (g) K p1 =1.49
Reversed ii) CO (g) + FeO(s) 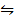 CO2 (g) + Fe(s) Kp2 =
CO2 (g) + Fe(s) Kp2 = 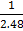
=0.403
Equation (i) + (ii) = (iii)
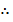 Kp3 =kp1 x kp2
Kp3 =kp1 x kp2
= 1.49 x 0.403
Kp3 = 0.6
1: When 1 mole of ethanoic acid is maintained at 25 0c with 1 mole of ethanol, 1/3 of ethanoic acid remain when equilibrium is attained. How much would have remained if 3/4 of 1 mole of ethanol had been used instead of 1mole at the same temperature.
CH3CH2OH + CH3COOH 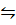 CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O
At start: 1mol 1mol 0 0
At time: 1-x 1-x x x
At equilibrium: 1 1- x =
1- x = 


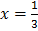
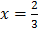
K c = 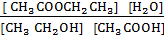
= 
= 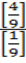
Kc = 4
CH3CH2OH + CH3COOH 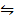 CH3COOCH2CH3 +H2O
CH3COOCH2CH3 +H2O
At start; 1mol 1mol 0 0
At time 1-x 1-x x x
At equilibrium (1 x 3/4)-x 1-x x x
Kc = 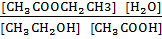
4 = 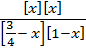 =
= 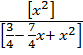
4 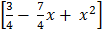 =X2
=X2
3- 7x + 4x2 = x2
3- 7X + 4X2 – x2 =0
3x2 + 3 – 7x = 0
3x2 -7x + 3 = 0
a b c
x =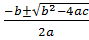
x =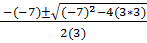
=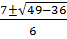
= 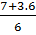
X= 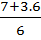 or x =
or x = 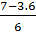
= 1.76 = 0.566
X cannot be 1.76
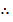 X = 0.566
X = 0.566
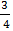 – 0.566 = 0.184 moles or 23/125 moles
– 0.566 = 0.184 moles or 23/125 moles
 0.184 moles of ethanol would have remained
0.184 moles of ethanol would have remained
2. The equilibrium constant (K c) for the reaction; 2HI (g) 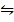 H2 (g) +I2 (g) is 0.02 of 400 0 c. If 2 moles of H2 and 1 mole of I2 were mixed together in a 1.0dm3 at 400 0c, how many moles of HI, I2 and H2 would be present at equilibrium.
H2 (g) +I2 (g) is 0.02 of 400 0 c. If 2 moles of H2 and 1 mole of I2 were mixed together in a 1.0dm3 at 400 0c, how many moles of HI, I2 and H2 would be present at equilibrium.
2HI (g) 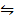 H2 (g) + I2 (g) K c = 0.02
H2 (g) + I2 (g) K c = 0.02
Reverse the equation above
H2 (g) + I2 (g) 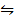 2HI (g) Kc =
2HI (g) Kc = 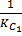
At start 2 1 0
At time 2-x 1-x 2x = 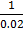 = 50
= 50
At equilibrium 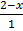
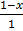
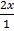
K c = 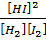
50 = 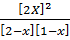
50 = 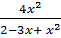
50 (2 – 3x + x2) = 4x2
100 – 150x + 50x2 = 4x2
100 – 150x +5 0x2 – 4x2 = 0
100 – 150x + 46x2 = 0
x=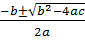
=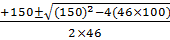
=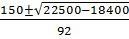
x= 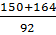 or x=
or x= 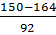
x= 2.33 x = 0.934
x cannot be 2.33
x = 0.934
At equilibrium:
Number of moles of H2 = 2- 0.934
=1.066moles
Number of moles of I2= 1-0.934
=0.066 moles
Number of moles of HI = 2 x 0.934
=1.868 moles
3. The equilibrium constant for the reaction; H2 (g) +Br2 (g) 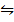 2HBr (g) at 1024K is 1.6 x 105. Find the equilibrium pressure of all gases if 10 atm of HBr is introduced into a second container at 1024K.
2HBr (g) at 1024K is 1.6 x 105. Find the equilibrium pressure of all gases if 10 atm of HBr is introduced into a second container at 1024K.
H2 + Br2 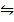 2HBr
2HBr