What is solubility ?
Is amount of substances dissolves in water completely so as give free ions.
Since the amount can be in gram (g) or in moles (mol) then the S .I unit for solubility is g/L or g/dm3
Also solubility can be expressed in mol / dm3 or mol/L . When solubility of substances is expressed in mol / dm3 and that is called molar solubility.
What is molar solubility ?
It is amount of solute in moles dissolve in a given dm3 of of solvent to give free ions.
When solubility is expressed in it’s S.I unit , that is the same as concentration in g /L or g /dm3 and when it is expressed as molar solubility , that is the same as molarity.
example. a) Define the following :
i) Solubility
ii) Molarity solubility
b) Calculate the following solubility in g /L 0.0004 M Na0H :-
i) Solubility is defined as the amount of a substance dissolve in water completely to give free ions.
ii) Molar solubility is the amount of solute dissolves in moles in a given dm3 of to give free ions.
Solution :-
i)Na0H Solubility = solubility in mol / dm3 x Mr
= 0.0004 x 40
= 0.016 g/L
The solubility of Na0H is 0.016 g /L
Solution:
ii) 2.7 x 10-3 mol /dm3 Ca(0H)2
Solubility = molar x Mr .
= 2.7 x 10 -3 x 74
= 0.1998 g/ L
The Solubility of Ca(0H)2 is 0.998 g/ L .
iii) 3.24 g of sodium chloride .
Solution:
Solubility = Molar Solubility x Mr
= 3.24 g/L x 58.5
58.5
= 3.24 g/L
These solubility of sodium chloride = 3.24 g/L
SOLUBILITY PRODUCT OF SPARINGLY SOLUBLE SALTS
Many salts which are referred to as insoluble do infact dissolve to a small/limited extent. They are called sparingly or slightly soluble salts.
In a saturated solution, equilibrium exists between the ions and undissolved salt.
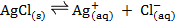
NOTE;
There is a limited number of ions that can exist together in water and this cannot be increased by adding more salts.
In a saturated solution of AgCl in contact with the ions, the equilibrium law can be applied
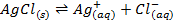
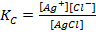
The concentration of solid is taken as constant at constant temperature
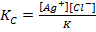
Kc + K = [Ag+][Cl–]
Ksp = [Ag+][Cl–]
Ksp = Solubility product constant.
By definition, Ksp is the product of maximum concentration of ions of sparingly soluble salt that can exist together in a solution at a given temperature.
OR
It is the product of concentration of all the ions in a saturated solution of sparingly soluble salts.
OR
Solubility product is the product of ions concentration in mol/dm3 of a certain solution raised to their stochiometric coefficient
Solubility product is denoted by Ksp
The unit for Ksp depends on the stochiometric coefficients of the respective ions of such particular substance
How to write Ksp expressions
In writing Ksp expressions one should look on
Ionization equation should be written correctly and balanced.
Stochiometric coefficients become powers of the respective ion.
Generally, for sparingly soluble salts
AxBy 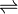 xAy+ + yBx-
xAy+ + yBx-
Ksp = [Ay+]x[Bx-]y
E.g. Write Ksp expression for the following equilibrium
(i) Al(OH)3 Al3++ 3OH–
(ii) Ag2CrO4 2Ag+ CrO42-
Solutions:
(i)Ksp = [Al3+] [OH–]3
(ii)Ksp = [Ag+]2 [CrO4
2-]
(iii)(Ca3(PO4)2 3Ca2+ + 2PO43-
Ksp = [Ca2+ ]3 [PO4
3-]2
Significance of Ksp
(i) Ksp value is used in the prediction of occurrence of precipitates if ions in the solution are mixed.
If concentrations of ions are enough to reach Ksp value, salt precipitation occurs
Determination of Solubility product from solubility measurements
The Ksp value of the salt can be determined from its solubility in moles per litre (mol/L)
When concentrations are given in any other units such as g/L, they must be converted to mol/L
Example 1
The solubility of AgI is 1.22 x 10-8 mol/L. Calculate the Ksp for AgI
Solution
AgI(s) 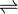 Ag+(aq) + I–(aq)
Ag+(aq) + I–(aq)
Each 1 mole of AgI that dissolves gives 1 mole of Ag+ and 1 mole of I– in solution, concentration of each ion solution is 1.22 x 10-8 mol/L.
Hence
AgI(s) 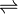 Ag+ + Cl–
Ag+ + Cl–
1.22 x 10-8 1.22 x 10-8
Ksp = [Ag+] [Cl–]
= (1.22 x 10-8)2
Ksp = 1.4884 x 10-16 mol2L-2
Example 2
PbCl2 dissolves to a slightly extent in water according to the equation
PbCl2(s)
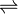 Pb2+(aq) + 2Cl–(aq)
Pb2+(aq) + 2Cl–(aq)
Calculate the Ksp for PbCl2 if (Pb2+) has been found to be 1.62 x 10 -2 mol l-1.
Solution
PbCl2 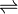 Pb2+ + 2Cl–
Pb2+ + 2Cl–
1.62 x 10 -2 1.62 x 10 -2 (2x 1.62 x 10-2 )
Ksp = [Pb2+] [Cl–] 2
= 1.62 x 10 -2 molL-1 x 1.0497 × 10-3 mol2L-2
Ksp = 1.7005 x 10 -5 mol3L-3 .
Example 3
The solubility of Pb(CrO4)is 4.3 x 10 -5 gl-1. Calculate the Ksp of Pb(CrO4)
(Pb =207, Cr = 52, O = 16)
Solution
PbCrO4 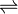 Pb2+ + CrO4
Pb2+ + CrO4
2-
4.3 x 10 -5gl-1 4.3 x 10 -5 gl-1 4.3 x 10 -5gl-1
To calculate the molar mass of (PbCrO4)
Pb(207) +Cr(52) +O(16)4=323gmol-1
323g → 1mole
4.3×10-5 → x
x=1.33×10-7mol
PbCrO4 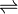 Pb2+ + CrO4
Pb2+ + CrO4
2-
1.33 x 10 -7 1.33 x 10 -7 1.33 x 10 -7
Ksp = [Pb2+] [CrO4
2-]
= (1.33 x 10-7)2
Ksp = 1.76 x 10 -14 M2
Example 4
100 ml sample is removed from water solution saturated with MgF2 at 18oC. The water is completely evaporated from the sample and 7.6mg of MgF2 is obtained. What is the Ksp value for MgF2 at 18oC
Solution
V =100 ml
m= 0.076g.
62g → 1 mole
0.076g → x
x = 1.22 x 10 -3 molel-1
MgF2 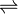 Mg2+ + 2F–
Mg2+ + 2F–
1.22 x 10 -3 1.22 x 10 -3 (2 ×1.22 x 10 -3 )
Ksp = [Mg2+] [F–] 2
= (1.22 x 10 -3) (2.44 x 10-3)
Ksp = 7.33 x 10 -9 moll-1