PRECIPITATION OF SPARINGLY SOLUBLE SALTS
If the equilibrium of sparingly soluble salts are approached by starting with ions in solutions and producing pure undissolved solute, then the process involved is precipitation reaction.
By definition, precipitation is the reaction where solid particles are formed by mixing ions in solution.
A substance will start precipitating as the reaction quotient (Q) becomes greater than the solubility product. Therefore, knowing the solubility product of a salt, it is possible to predict whether on mixing the solutions of ions precipitations will occur or not and what concentration of ions are required to begin the precipitation of the salt.
As for Ksp, Qsp are also given by
Qsp = [Ay+]x [Bx-]y
Where [Ay+] and [Bx-] are the actual ions concentrations and not necessary those at equilibrium
If Qsp = Ksp the system is at equilibrium.
If Qsp < Ksp The solution is unsaturated and precipitation does not occur.
If Qsp > Ksp the solution is super saturated and precipitations occurs. REASON : So as to maintain Ksp value.
Example 1
The concentration of Ni 2+ ions in a solution is 1.5 x 10 -6 M. If enough Na2CO3 is added to make the solution 6.04 x 10 -4 M in the CO32- ions will the precipitates of Nickel carbonate occur or not?
Ksp for Ni2+ 6.6 x 10 -9 M2
Solution
NiCO3 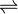 Ni2+ + CO32-
Ni2+ + CO32-
Qsp = [Ni2+][CO2-]
= (2 x 1.5 x 10 -6)2 (6.04 x 10 -4)
Qsp = 5.4 x 10 -4 M2
Qsp >Ksp (Precipitation will occur)
Example 2
Predict whether there will be any precipitates on mixing 50cm3 of 0.001m NaCl with 50cm3 of 0.01m of AgNO3 solution. (Ksp for AgCl 1.5 x 10 -10M2)
Solution
Concentration of Cl–
NaCl 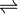 Na+ + Cl–
Na+ + Cl–
0.01 0.001 0.001
0.001 → 1000cm3
x → 50cm3
x = 5 x 10 -5 moles (These are in 100cm3 since we added 50cm3 ofAgNO3)
Now 50 x 10 -5 moles → 100 cm3
x → 1000cm3
x = 5 x 10 -4 moll-1
Now for Ag+
AgNO3 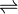 Ag+ + NO3-
Ag+ + NO3-
0.01m 0.01m 0.01m
0.01 moles → 1000cm3
x → 50cm3
x =5 x 10 -4 moles
5 x 10 -4moles → 100cm3
x → 1000cm3
x = 3 x 10-3 mol l–
AgCl 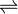 Ag+ + Cl–
Ag+ + Cl–
Qsp = [Ag+][Cl–]
Qsp = (5 x 10 -3)(5 x 10-4)
Qsp = 2.5 x 10-6 M2
Qsp > Ksp
Precipitation will occur.
Example 3
If a solution contains 0.001M CrO42- , what concentration of Ag+ must be exceeded by adding AgNO3 to the solution to start precipitation Ag2CrO4? Neglect any increase in volume due to additional of AgNO3 (Ksp of Ag2CrO4 9.0 x 10-12M2)
Solution
Ag2CrO4 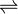 2Ag+ + CrO42-
2Ag+ + CrO42-
Ksp = [Ag+][CrO42-]
9 x 10 -12 = [Ag+]2 0.001
[Ag+]2 9 x 10 -9
[Ag+] = 9.48 x 10-5 M
Example 4
The Ksp value of AgCl at 18°C is 1 x 10 -10 mol2l-2, What mass of AgCl will precipitate if 0.585g of NaCl is dissolved in 1l of saturated solution of AgCl.
(Ag = 108, Na = 23, Cl = 35.5)
Solution
AgC l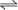 Ag+ + Cl–
Ag+ + Cl–
Ksp = [Ag+][Cl–]
1 x 10 -10 = S2
S=1 x 10-5M
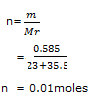
n = 0.01moles
AgCl 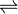 Ag+ + Cl-
Ag+ + Cl-
S S 0.01 + S
0.01 S ≈ 0.01
Ksp = [Ag+][Cl–]
1×10 -10 = S(0.01)
S = 1 x 10 -8 moll-1 (Solubility has decreased)
To find the amount which has precipitated,
S = 10-5 – 10-8
S = 9.99 x 10 -6M
9.99 x 10 -6 mole → 1l
143.5g → 1 mole
9.99 x 10 -6
m = 1.43 x 10 -3g of AgCl
Question 1
Should precipitation of PbCl2 be formed when 155cm3 of 0.016M KCl is added to 245cm3 of 0.175M Pb(NO3)2? Ksp is 3.9 x 10 -5
Question 2
If concentration of Zn2+ in 10cm3 of pure water is 1.6 x 10 -4 M. Will precipitation of Zn(OH)2 occur when 4mg of NaOH is added.(Ksp for Zn(OH)2 is 1.2 x 10 -17)
Question 3
A cloth washed in water with manganese concentration exceeding 1.8 x 10 -6 may be stained as Mn(OH)2 (Ksp 4.5 x 10 -14) . At what pH will Mn2+ ions concentration be equal to 1.8 x 10 -6M
Solution 1
Mn(OH)2  Mn2+ + 2OH–
Mn2+ + 2OH–
Ksp = [Mn2+][OH–]2
4.5 x 10 -14 = 1.8 x 10 -6 [OH–]2
[OH-]2 = 2.5 x 10 -8
[OH-] = 1.58 x 10 -4 M
pOH = -log[OH–]
= -log(1.58 x 10-4)
p(OH) = 3.801
pH + pOH =14
pH = 14 – pOH
=14 – 3.801
pH = 10.199
Solution 2
We find concentration of OH- in NaOH
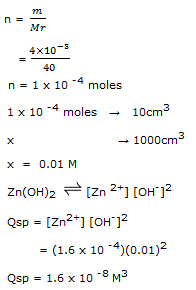
Qsp > Ksp Hence precipitation will occurs