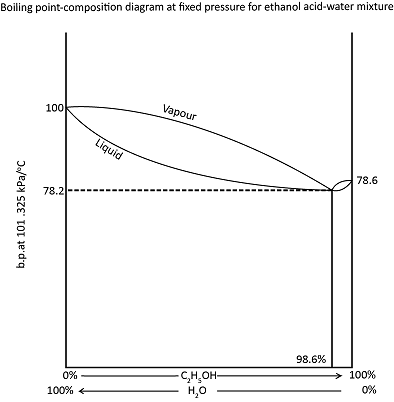
BOILING POINT COMPOSITION DIAGRAM WITH A MINIMUM
Ethanol and water give a vapour pressure composition diagram with a maximum. The corresponding boiling point composition diagram, with a minimum is shown in figure below. It is not possible to get a complete separation of ethanol and water by fractional distillation. A mixture containing more than 95.6 percent ethanol can be separated
into pure ethanol and a minimum boiling point mixture, with a composition of 96.5 percent ethanol. A mixture containing less than 96.5 percent ethanol can be separated int pure water and the same water and the same boiling point mixture.
Water with propanol or pyridine and ethanol with tri-chloromethane or methyl benzene, also give minimum boiling point mixtures.
SEPARATION OF AZEOTROPIC MIXTURES
An azeotropic mixture may have either a maximum or a minimum boiling point but at any one pressure, it has a fixed composition.
It is a unusual for this composition to correspond with that of any sample chemical formula for the mixture, and there is definitely no compound formation because the composition of the mixture does not depend on pressure.
Moreover, the mixture can be separated into its component parts fairly easily. Such separation can be brought about by the following methods.
a) By distillation with a third component
The azeotropic mixture of ethanol and water contains 95.6 percent of alcohol at normal atmospheric pressure. If benzene is added distillation yields, first a ternary azeotropic mixture of ethanol, water and benzene, then a binary azeotropic mixture of ethanol and benzene, and finally absolute ethanol.
b) By chemical methods
Quicklime may be used to remove the water from an azeotropic mixture of ethanol and water. Concentrated sulphuric acid will remove aromatic or unsaturated hydrocarbons from mixtures with saturated hydrocarbons in the refining of petrols and oils.
c) Absorption
Charcoal or silica gel may absorb one of the components.
d) Solvent extraction
One component can be extracted by a solvent.
PARTIALLY MISCIBLE LIQUIDS
Critical Solution temperature.
Phenol and water are completely miscible, forming one solution, above 66oC, but two immiscible solutions may from below that temperature, depending on the composition of the mixture. One of the solutions will be a solution of phenol in water, the other a solution of water in phenol.
They called conjugate solutions
The effect of composition and temperature is shown in a temperature-composition diagram below. The temperature above which phenol and water are always completely miscible is known as the upper critical solution temperature. At any point above the curve there will only be one layer, i.e. one solution. Below the curve, two layers will-always form and the curve will give the compositions of the two conjugate solutions making up the two layers. A mixture of 50 per cent phenol and 50 per cent water, for example, at 50 °C, will form two layers whose compositions are given by A and B. The line YZ is known as a tie-line. The ratio YX/XZ is equal to the ratio of the mass of the phenol layer (of composition B) to that of the mass of the aqueous layer (of composition A).
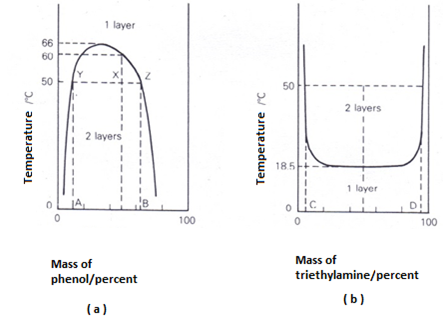
The complete miscibility of phenol and water with increasing temperature comes about because their mutual solubilities increase as the temperature does. The curve in Fig.(a) can be regarded as made up of two halves, one being the solubility curve of water in phenol and the other the solubility curve of phenol in water.
With triethylamine and water the mutual solubilities decrease as the temperature is increased. This leads to a temperature-composition diagram with a lower critical solution (or consolute) temperature of I8,5°C (Fig. (b)). A 50:50 mixture will be completely miscible at 10 °C but will separate into two layers, with compositions C and D, at 50 °C.
Mixtures of nicotine and water are very unusual as they have both an upper (208 °C) and a lower (61 °C) critical solution temperature.
Conjugate solutions have the same total vapour pressure and the same vapour composition; that is why they can coexist together.
REVIEW QUESTIONS
1. Some solution are ideal while others are not briefly explain what do you understand by this statement
2. How do ideal gases differ from ideal solution?
3. Define the following
i) Mole fraction of that liquid
ii) Ideal solution
iii) Briefly explain how Raoult’s law becomes feasible
4. Vapour pressure of methyl alcohol and ethyl alcohol at 20oc are 94 mmHg and 44mmHg respectively. If 20g of ethyl alcohol and 100g of ethyl alcohol are mixed. Calculate
a) Partial pressure of each in a mixture
b) Total pressure of the mixture
c) % composition of each alcohol in a mixture
5. Define the following terms
i) Vapour pressure
ii) Partial Vapour pressure
6.a) Define the following
i) Ideal solution
ii) Non ideal solution
b) State the Raoult’s law partial pressure
Answer
a) i) Ideal solution are these solution that do obey all assumption of Raoult’s law of partial pressure
ii) Non ideal solution are these solution that do not obey some or all assumption of Raoult’s law of partial pressure
b) i) Raoult’s law of partial pressure states that “The saturated vapour pressure of each component in a mixture is equal to the product of mole fraction of that component and its pure vapour pressure”
7.a) ii) Raoult’s law
ii) Partition law
b) The ideality of a solution is approached when it is made more dilute explain
c) 10 g of methanol give an ideal solution when mixed with 50g of ethanol. If the vapour pressure of methanol and ethanol at the same temperature are 6265 Pa and 2933 Pa respectively
Calculate
i) The partial pressure exerted by each component in the mixture
ii) The component of the vapour
8.a) State
i) Boyl’s law
ii) Charles’s law
iii) Avogadros law
b) SO2 used in manufacture of sulphuric acid is obtained from sulphide ore
4 Fe2(s) + 11O2(g) 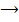 2Fe2O3(s) + 8 SO2(g)
2Fe2O3(s) + 8 SO2(g)
Find the mass of oxygen in grams reacting when 75 litres SO2 is produced at 1000C and 1.04 atm.
9. What is azeotropic mixture?
Azeotropic mixture is the mixture of two different components with constant vapour pressure and boiling point and it cannot be separated by fractional distillation.
10. What is azeotropic point
Azeotropic point is the point in temperature composition graphic which shows the azeotropic temperature and its composition
11. What is azeotropic temperature?
Azeotropic point is the temperature is the temperature at which azeotropic mixture tend to boi
The temperature composition graph of non ideal solution shows some deviations from ideal behaviour deviation
i) Positive deviation
ii) Negative deviation
iii) Boiling point composition diagram which undergo Positive deviation
12. a) Define the following terms
i) Azeotropic mixture
ii) Azeotropic point
iii) Boiling point
iv) Azeotropic composition
b) Liquid Q and R from a non ideal solution. If liquid Q boil at 100oC and R boil at 43% less than that of Q. On boiling the azeotropic mixture was formed at 56% composition by mass liquid Q and boil at 51oC
i) Plot the temperature composition graph
ii) What type of deviation is shown by your graph?
13. a) What do you understand by the following terms
i) Non ideal solution
ii) Azeotropic solution
b) How does non ideal solution deviate from ideal solution?
c) The mixture container the following water and nitric acid which boil at 86oC . The composition of azeotropic is 68% by mass nitric acid
i) Plot the boiling point curve which represent above data
ii) Account for the distillated and residue on distilling the mixture contain 50% by mass water
iii) Account for the distillated and residue on distilling the mixture contains 78% by mass HNO3. If azeotropic temperature is 12oC
iv) Is the deviation positive or negative? Why?
v) 20% by mass HNO3 . Account for the distillated at the point.
14. i) Raoult’s law states that
The saturated vapour pressure of a component in a mixture is equal to the product of mole fraction of that components and its partial vapour pressure
ii) Partition law state that
When a solute is added to two immiscible solvents it distribute itself between the two solvent until the ratio of concentration of solute in one solute to another is constant, provided that solute remains in the same molecular state in both solvent and temperature is constant
b) The ideality of a solution is approached when it is made more dilute because the attractive or repulsive forces between solvent and solute molecules become weaker and cause the gas to obey assumption Raoult’s law .
As a solution become more dilute
* In start stronger forces, grater deviation from Raoult’s law , solution become more constant