4. OSMOTIC PRESSURE
What is osmotic pressure ?
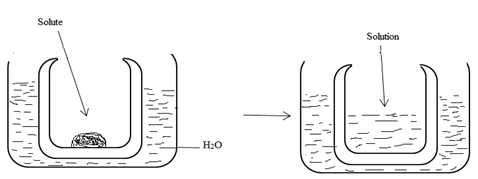
Osmotic pressure: Is the force per unit area which occur as the results of solvent to flow From low concentration to the high concentration of solute through a semi permeable membrane .
Osmotic pressure causes the osmosis to take place.
Osmosis: Is the tendency of solvent molecular to migrate from the region of low concentration to the region of high concentration of solute.
Consider the simple experiment below
Factors affecting the Osmotic pressure of the substance
The Osmotic pressure of the substance affected by the two factors
i) Concentration or volume of the solvent.
ii) Temperature
i) CONCENTRATION OR VOLUME OF THE SOLVENT
The osmotic pressure of the substance varies directly proportional to the concentration and inversely proportional to the volume .
i.e An increase in concentration of the solution will cause an increase in osmotic pressure of the solution.
Also increases in osmotic pressure cause the decrease in volume.
ii) TEMPERATURE
The temperature varies directly proportional to the osmotic pressure as long as the temperature do not exceed the optimum temperature of the semi permeable membrane.
The effect of two factors above are explained by Vant- hoff’s laws of osmotic pressure.
VANT – HOFF’S LAWS OF OSMOTIC PRESSURE
Vant – hoff tried to put forward his laws which explain the variation of osmotic pressure with concentration and temperature.
There are two laws developed, These are:
i) Vant- hoff”s first law of osmotic pressure.
ii) Vant – hoff’s second laws of osmotic pressure.
i) VANT – HOFF’S FIRST LAW OF OSMOTIC PRESSURE
It states that;
“The osmotic pressure of the solution is inversely proportional to its volume”
Let the osmotic pressure be denoted by π
From 1st law
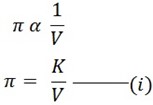
ii) VANT – HOFF’S SECOND LAW OF OSMOTIC PRESSURE
It states that;
“The osmotic pressure of the solution is directly proportional to temperature”
From 2nd law
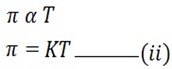
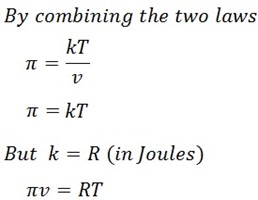
This is for 1 mole, But for ‘n’ mole the formula will be
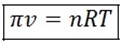
Example1
7.85g of the compound Y having the empirical formula of C5H4 was dissolved in 301g of Benzoic (C6H6) . If the freezing point of the solution is 1.05oC below that of pure benzene.
i) Determine the molecular mass of Y
ii) Find the molecular formula of Y if kf for C6H6 is 5.12oC kg mol-1
Solution
Mass of solute = 7.85g
Mass of solvent = 301g
F . P depression ΔT = 1.05oC
Kf of solvent = 5.12oC kgmol-1
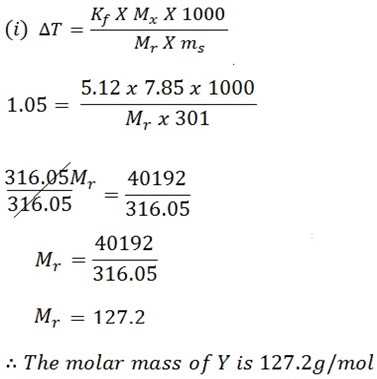
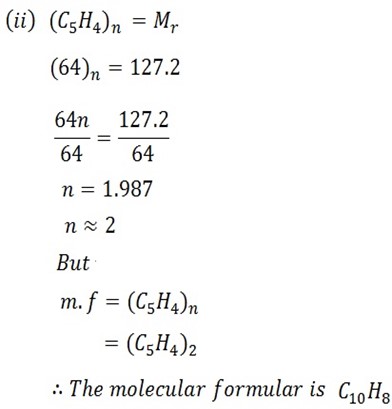
Example 2
Calculate the molar mass of solute given that 35g of solute dissolved in 1dm3 has osmotic pressure of 5.51 N/M2 at 20oC. (R = 8.314 Jmol-1k-1)
Solution
Mass of solute = 35g
Volume of solution = 1dm3
Osmotic pressure π = 5.51
Temperature T = 20oC + 273k
= 293k
πv = nRT
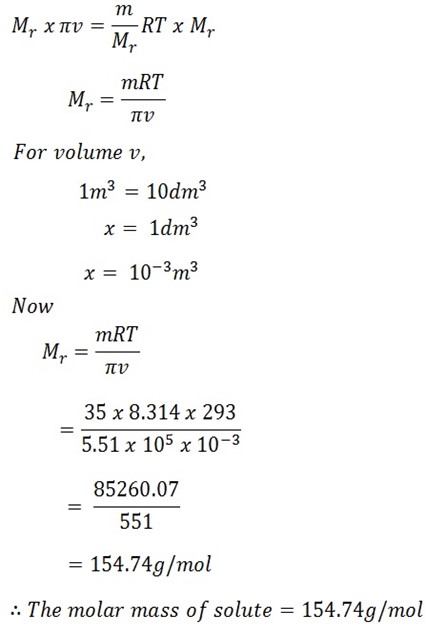
Example 3
a) Define
i) Osmosis
ii) Osmotic pressure
b) Differentiate between osmosis and diffusion
Answers
a) i. Osmosis : Is the tendency of a solvent molecules to move from the region of law concentration to the region of high concentration of solute.
ii. Osmotic pressure : Is the force per unit area which occur as the result of solvent to flow from low concentration to the high concentration of solute through a membrane.
b) Osmosis is the movement of solvent molecules from the region of low concentration to the region of high concentration of solute through a semi- permeable membrane while Diffusion is the movement of gases molecules from the region of high concentration to a region of low concentration .
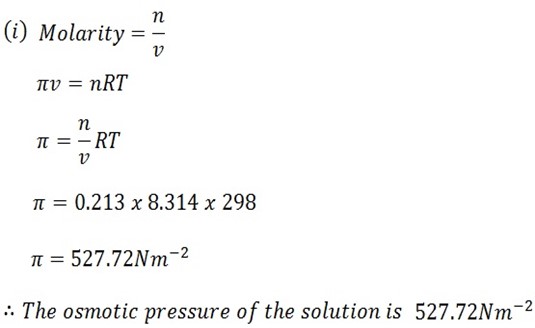
c) Calculate the osmotic pressure of the following solution at 25oC
i) Sucrose solution of concentration 0.213M (527.72 N/m2)
ii) A solution of glucose (C6H12O6) made by dissolving 144g/dm3 . (1.1982 x 106 N/m2)
c) Solution
Temperature T = 25oC + 273
= 298k
Molarity = 0.213M
(ii) Molar mass of glucose = 180
Concentration = 144g/dm3 = 144000g/m3
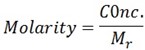
= 144000 ÷ 180
= 800M
Osmotic pressure π = MRT
= 800 x 8.314 X 298
= 1.982 X 106N/M2
∴ The osmotic pressure of a solution = 1.982 X 106 N/M2
Example 4
Calculate the osmotic pressure of the solution containing 12g of C6H12O6 in 300g of water at 20oC.
R = 8. 314 Jmol -1k-1(54.13 X 102 N/m-2).
Solution
Mass of solute = 12g
Mass of water = 300g hence volume = 300cm3
Temperature T = 20o + 273
= 293k
But
1m = 100cm
1m3 = 1003cm3
X = 300cm3
1003
X = 3 X 10 -4
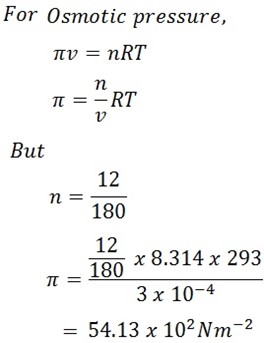
Example 5
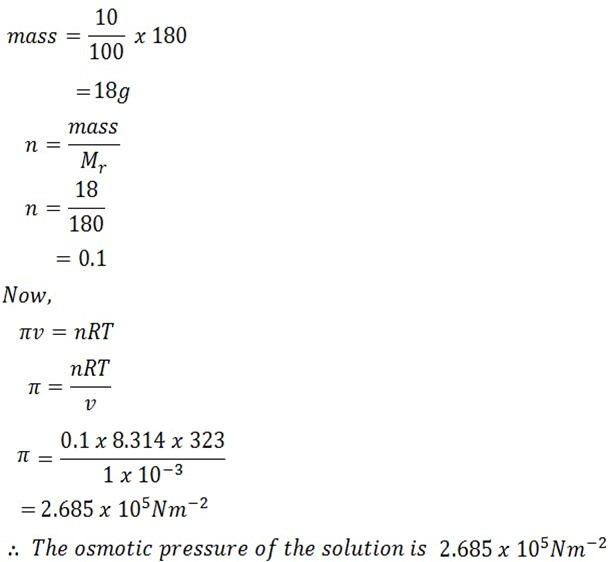
Calculate the osmotic pressure of 10% glucose solution at 50oC R= 8.314 Jmol-1k -1
Solution
Temperature T = 50oC + 273
= 323k
Mass = 10% glucose
Molar mass of glucose = C6H2O6
= 180
Example 6
37.44g of haemoglobin, the protein of red blood cell which carries the oxygen in the blood, were dissolved in a dm3 of water.
The solution formed had an osmotic pressure of 1.37 kPa at body temperature of 37oC.
Determine the molar mass of haemoglobin.
Solution
Mass of solute = 37.44g
Volume = 1 dm3
Temperature = 37oC + 273k
=310k
Osmotic pressure = 1.37 kPa X 1000
= 1370Pa
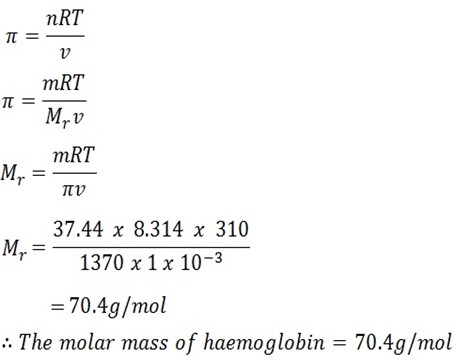
Example 7
A solution containing 10g of solute A in 300. Of water has an osmotic pressure of 375mm of 25oC. What will be the osmotic pressure be dissolving 2.55g of solute A in 50g of water? R = 0.0821 atm mol-1 k -1 L.
Solution
Mass of solute A MA =10g
Volume of solvent = 300cm3 = 0.3m3
Osmotic pressure = 375mmHg
1 atm = 760mmHg
X = 375mmHg
X = 375
760
= 0.49
Temperature T = 25oC + 273k
= 298k
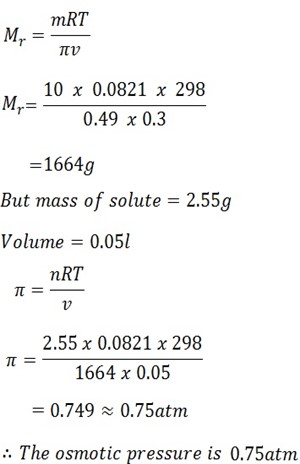
COLLIGATIVE PROPERTIES OF ASSOCIATIVE AND DISSOCIATIVE SOLUTE
Colligative properties depends on vant hoff’s factor . Vant hoff factor is the ratio of observed colligative properties to that of calculated colligative property.
OR
Vant hoff factor is the ratio of experimental colligative property to that of theoretical colligative property.
VANT HOFF FACTOR
Vant hoff’s factor is denoted by ‘g’ or ‘l’ and therefore

Vant’s hoff factor is obtained when the solute added is not completely ionized (dissociated).
Also some solutes, when added in the solvent tend to associate.
The phenomena of associating or dissociating of solute is explained in terms of degree (degree of association or dissociation).
The degree of dissociation is
Define:
The degree of dissociation is the percentage or fraction of moles of ions which have gone into the solution.
It is denoted by α
The degree of dissociation is related to Vant hoff’s factor and the number of ions formed by the solute dissolved.
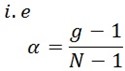
Where by;
α is the degree of dissociation
g is the Vant hoff’s factor
N is the number of ions
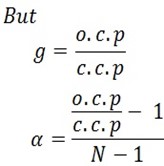
Example 1.
Calculate the N values for the ionization of the following compounds
Solution
i) Al ( OH)3
Ionization equation
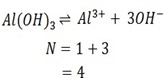
ii) FeCl3
Ionization equation
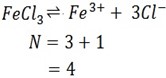
iii) BaCl2
Ionization equation
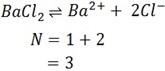
iv) NaCl
Ionization equation
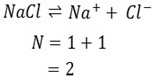
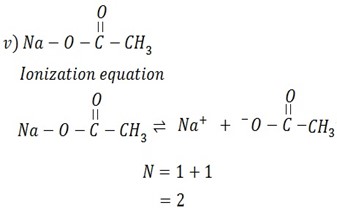
NOTE
The degree of dissociation can be expressed terms of
i) Percentage%
ii) Decimals
When it is expressed in percentage it cannot exceed 100% and
When it is expressed in decimal it cannot exceed 1.00
Also when solute is ionizing completely its degree of dissociation become 100% OR 1.00
Example 2.
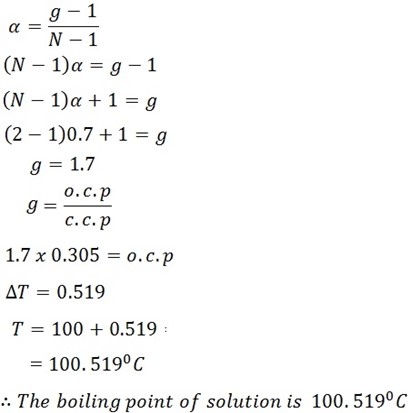
What will be the boiling point of the solution containing 2.4g NaCl in 250cm3 of water . it an aqueous solution of NaCl is 70% Dissociation.
(kb water = 1.86)
Solution
Mass of solute mx = 2.4g.
Mass of solvent ms = 250g.
Kb = 1. 86.
α = Degree of elevation 70% or o.7http://192.168.137.101/tz/cexam.php?MASTexam=586
Mrx = NaCl
= 23 + 35.5
= 58.5 g/mol
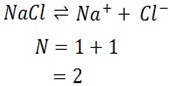
Δ T = Kb x m x 1000
ms X Mrx
= 1.86 x 2.4 x 1000
250 x 58.5
= 0.305oC
c.c.p = 0.305oC