Soil – may be defined as the unconsolidated mineral on the top layer of the earth’s crust that serves as a natural medium for the growth of land plants.
SOIL COLLOIDS:
Definition: Soil Colloids – are very small organic and inorganic parties present in the soil which are responsible for potential fertility of the soil determine the physical and chemical properties of the soil.
1. How soil colloids are formed
· As soil is formed during weathering processes, some minerals and organic matter are broken down to extremely small particles.
· Chemicals changes further reduce these particles until they cannot be seen with the naked eyes.
· The very smallest particles are what we call soil colloids.
2.
Types of soil colloids
Soil collards may be
a) Inorganic colloids
– Clay minerals (layer silicate clay).
– Iron and Aluminium oxide clays.
– Allophane and amorphous clays.
b) Organic colloids
– Includes highly decomposed organize matter called humus.
– Organic colloids are more reactive chemically and generally have greater influence on soil properties.
Therefore the four major colloids present in the soil are,
i) Layer silicate clay
-These are most important silicate known as phyllosilicates (life-like).
– They comprised of two kinds of horizontal sheets, one dominated by silicon and other by aluminium magnesium.
ii) Iron and Aluminium
These are remnant material which remain after extensive teaching due to its low solubility these are sesquioxides
Sesquioxides are either Al oxide or iron (iii) oxide contaminated with Al(OH)3 or Fe(OH)3
iii) Allophane and other armophous minerals
– Mainly these silicates are mixture of silica and alumina.
– They are amorphous in nature.
iv) Humus
-Humus is armophous, dark-brown to black nearly insoluble in water but more soluble in dil. Alkali e.g NaOH, KOH solution.
– It consists of various chain loops of linked carbon atoms.
– Humus is a temporary intermediate product left after considerable decomposition of plant and animal remains.
-Humus contains partially dissociated enolic, carboxyl and phenolic groups.
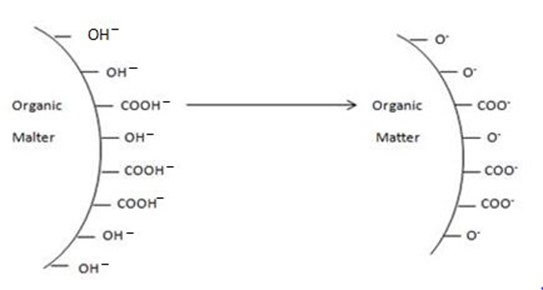
PROPERTIES OF SOIL COLLOIDS
-Includes
i. Surface area
ii. Electric change (surface charge )
iii. Ion exchange (adsorption of cation)
i) Surface area.
Because of their small size, all soil colloids expose a large external surface area per unit mass.
· The external surface area of 1g of colloidal clay is at least 1000 times of 1 g of colloidal clay is at least 1000 times that of 1g of coarse sand.
ii) Electric charge
· Soil colloids surface, both external and internal characteristically carry +ve and or -ve charge.
· Most soil colloids the +ve charge predominate.
· Both organic and inorganic soil colloids when suspended in water carry a negative charge.
Where the negative charge on colloidal particles comes from?
· Negative charge on clays comes from
i) Ionizable hydrogen ions.
ii) Isomorphous substitution.
i) Ionizable hydrogen ions are hydrogen ions from hydroxyl group on clay surfaces
· The O-H bond from Al –OH or Si – O –H portion of clay heterolytically breaks and ionizes to give H+ leaving unneutralized negative charge on oxygen.
· Presence of strong alkaline solution activates the clearage of O-H bond yielding H+ which will combine with OH– from strong alkaline solution in the neutralization reaction.
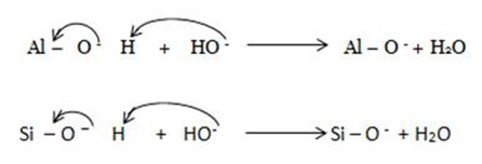
Hence soil colloids becomes more negative in more alkaline solutions.The same applies to organic colloids which also contain OH in enolic, phenolic or enol.
ii) Isomorphous substitution.
This is due to the substitution of one ion for another of similar size often with lower change
iii) Ion exchange (Adsorption of cations)
As soil colloids posses negative charge, they attract the ions of an opposite charge i.e positively charge ions to the colloidal surfaces. The attraction of cations such as H+, Ca2+, and Mg2+to colloid surface leads to formations of an ionic double layer. The outer layer is made up of a swarm of rather loosely held cations attracted to the negative charged colloidal surface.
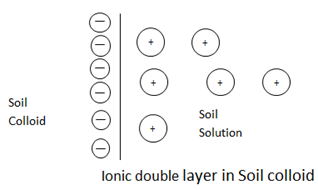
ION EXCHANGE IN A SOIL
Colloids are primarily responsible for chemical reactivity in soil
Each colloid has net negative charge thus making possible for the colloid to attract and hold positively charged particles.(cations) like Na+, H+, Ca2+ and Mg2+
– Thus there are cations attached to colloids and in the soil.
– When one of the cations in the soil solution replaces one of the cations on the soil colloids cation exchange is said to take place.
– This exchange only take place when the cations in the soil solution are not in equilibrium to the cations on the soil colloid.
Definition
Ion exchange :
Is reversible reaction which involves an interchange of ions between ion in soil solution and another ion or surface of soil colloid.
It can be anion exchange or cation exchange.
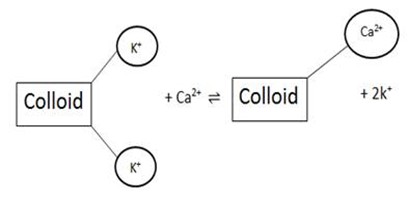
Cation exchange:
Is the interchange between a cation in a solution and another cation on surface of any negatively charged material such as day or organic matter.
In the soil:Cation exchange is the interchange between cation in a soil solution and another cation on the surface of soil colloid.
e.g
Anion exchange
· Soil colloids being negatively charged cannot attract and hold negatively charged particles (like charged repels) like SO4 and NO3
Why? Nitrate is more leached from the soil than ammonium.
· This is because nitrate (NO3) has negative charge like soil colloids. So NO3 is not held by the soil solution to be leached under rainfall conditions.
· NH4 being positively charged is attracted and held by soil colloids and hence it become difficult for NH4 to be leached.
Mechanism of ion exchange in the soil.
· Ion exchange in the soil is well explained by electron –kinetic theory of ion exchange.
According to the theory:
The negative and positive charge associated with soil colloids (clay minerals and organic matter) are balanced by electrostatic attraction of cations and anions respectively.
The balancing ions are turned as EXCHANGEABLE CATIONS OR ANIONS.
· Exchangeable cations and anions form outer sphere complexes with charged surfaces in which water of hydration exist between the charged ion and the oppositely charged colloids.
· Thus the adsorbed cations and anions are said to being state of oscillation forming a diffuse double layer.
· Due to these oscillations, some of the ions move away from the surface of the colloid micelles.
· In presence of the solution of an electrolyte an ion of the soil solution slips in between the inner charged layer and the outer oscillating ion.
· The ion in the soil solution is now adsorbed on the colloid micelles and the surface in remains in solution as an exchange ion and hence the ion exchange occurs.
Factors affecting composition of exchangeable ions in the ion exchange
Explain the factors affecting composition of exchangeable ions in the soil.
i. Strength of adsorption
ii. Relative concentration of the ion in the soil solution.