CATION EXCHANGE CAPACITY (CEC)
– Is the maximum quantity of total cations of any class, that a soil is capable of holding at a given PH value, available for exchange with soil solution.
– It is a measure of the quantity of the negatively charged sites on soil surfaces that can retain positively charged ions by electrostatic force.
Significance of CEC
CEC is useful in the following ways:
It is a measure of soil fertility
-The more cation exchange capacity a soil has the more likely the soil will have fertility.
It is a measure of nutrient retention capacity
-Soil with large value of CEC has large nutrient retention capacity.
It is a measure of the capacity of soil to protect ground water from cation contamination.
-Soil with large CEC has good ability to protect ground water from cation contamination as it exerts more resistance for its cations to be leached away.
Expression of CEC value
It is expressed in terms of number of equivalents (or more specifically as number of millequivalents) of cations per 100 grams of dry soil written as:
e.g 100g (meq /100g)
No of equivalents= 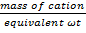 ————- (i)
————- (i)
Equivalent 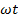 means the mass of cation that will replace (exchange) 1g (1mole) of H+.
means the mass of cation that will replace (exchange) 1g (1mole) of H+.
E.g
23g of Na+ (i.e 1mole of Na+) exchange 1g of 1+ and hence Na+ has equivalent wt of 23g.
4og of Ca2t (I mole of Ca2+) exchange 2g (2moles of H+ which means 20g 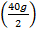 of Ca2+ exchange 1g
of Ca2+ exchange 1g 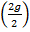 of H+
of H+
Hence Ca2+ have equivalent wt of 20.
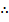 equivalent
equivalent 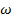 t=
t= 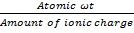 ——— (iii)
——— (iii)
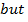
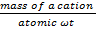 = no of moles of the cation
= no of moles of the cation
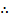 number of equivalents =
number of equivalents =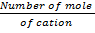 x Amount of ionic charge
x Amount of ionic charge
Where no of mill moles = No of moles x 1000
No of mill moles =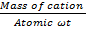 x 1000
x 1000
= 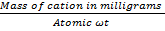
Factors affecting value of cation exchange capacity.
Cation exchange capacity is affected by the following factors:
– Amount of clay (soil texture)
– Type of clay
– Soil organic matter
– PH of the soil.
i) Amount of clay in soil
A high silicate- clay soil hold more exchangeable cations than a low silicate clay soil and hence have greater CEC value.
Silicate clay (Si – (OH) easily lose H+ off Hydroxy group in basic medium resulting into net negative charge i.e the greater the –ve charge the greater the CEC
(ii) Clay types
Different clay types have different CEC value due to difference in surface area.
(iii)Soil Organic Matter
Organic matter in the Soil, also are negative charged. Therefore
– High – Organic Soil have greater CEC value than a low organic Soil.
– On another hand if an organic matter continue to decay, the CEC tends to decrease with the decomposition.
– They can retain more cations.
Therefore organic matter particles have greater CEC than clay particles.
(iv) PH of the soil.
PH affects CEC of colloids that are charged based on hydroxyl group instead isomorphous substitution.
Under acidic (low PH ) condition.
H+ are in excess resulting to less ionization of the colloids, less negative charge and hence low CEC value.
Under basic (High PH ) condition.
OH– are in excess resulting to more ionization of the colloids , more negative charge on the colloids surface and hence high CEC value.
PERCENTAGE BASE SATURATION
Acid cations and Base cations
Acid cations are exchangeable cations (mainly (H+ and AI3+) which tend to acidify the soil
– They are also known as exchangeable acids
– In very acidic soil, H+ and AI3+ dominates other adsorbed cations.
– The acidity of AI3+ is explained by its cationic hydrolysis according to the following equation:
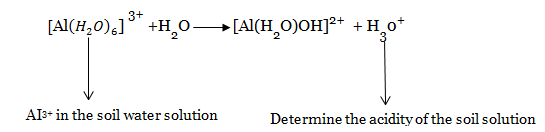
· Base cations (or exchangeable bases) are exchangeable cations which are capable of neutralizing soil acidity . Common exchangeable bases are Ca2+, Mg2+, K+ and Na+
Base saturation
Base saturation is the fraction of exchangeable cations that are base cations.
-It is expressed as percentage and hence the name percentage base saturation.
Percentage base saturation =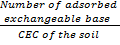 x 100%
x 100%
Relationship between percentage base saturation and percentage acid saturation
Percentage base saturation + percentage acid saturation
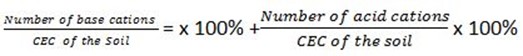
=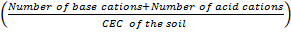 x 100%
x 100%
but number of base cations + Number of acid cations= Total number Of exchangeable cations = CEC of the soil
=  x 100% =100%
x 100% =100%
Therefore
Percentage base saturation + percentage acid = 100% Saturation.
Q. 1. (a) Define the following terms as applied to soil:
(i) Cation Exchange Capacity
(ii) Percentage Base saturation
(iii)Salinity
(b) A soil sample has a CEC of 25meq per 100g of 200mg of soil sample were shaken with 40c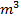 of 0.1M HCl. After filtering and washing the soil the filtrate and washing were titrated against NaOH solution, 24.0cm3 of 0.1 M NaOH were required for complete neutralization. Calculate the percentage base saturation of the soil sample.
of 0.1M HCl. After filtering and washing the soil the filtrate and washing were titrated against NaOH solution, 24.0cm3 of 0.1 M NaOH were required for complete neutralization. Calculate the percentage base saturation of the soil sample.
(c) A soil sample (20g) was analyzed and found to contain 0.0015g of calcium, what is the concentration of Ca in the soil sample in meq/100g of soil.
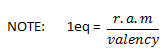
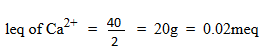
1eq of Na + 23g = 0.023meq
ANSWERS:
(b) Solution:
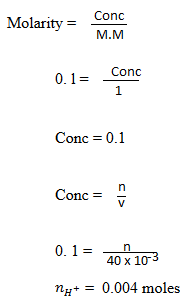
Remaining HCl will react with NaOH hence HCl was in excess
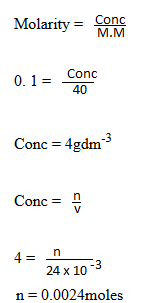
Used = 0.004 – 0.0024
= 0.0016moles of H+ (No of moles which attached themselves to the soil colloid)
Now we know 1eq = 1 mole of H+
0.0016 eq was in 200g of soil
? 100g
0.0016 e.g. = 200
? = 100g
8 x 10-4 x 1000 = 0.8meq/100g
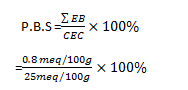
P. B.S. = 3.2%
Example 4
A Soil test shows the following:
a) Calculate the CEC of the Soil.
b) Calculate the percent base saturation of the Soil
c) Calculate the percent aluminium saturation of the soil
SOLUTION:
a) CEC of the Soil
= number of exchangeable base cations + number of exchangeable acid cations.
= (9.9 + 2.1 + 2.0 + 7.6 + 0.6 + 1.0) meq = 22.3 meq
Hence CEC of the soil is 22.3 meq
b) From a given cations, are acid cations
= (22.3 – (0.6 + 7.6)) = 14.1
(Recall: CEC = Number of exchangeable base cation + Number of exchangeable acid cations, and therefore number of exchangeable base cation = CEC – Number of exchangeable acid cations)
Using; Percent base saturation
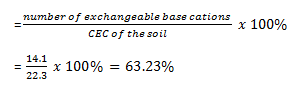
Hence percent base saturation is 63.23
Percent aluminium saturation
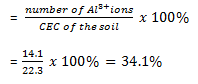
Hence aluminium base saturation is 34.1%
SOIL REACTION – It is the acidity or alkalinity of the soil. The soil reaction can be acidic, neutral or alkaline due to the soil solution. It is
measured in PH using electrometric methods (PH – meter). All soil PH range from 4 to 8. Soils with PH 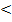 4 generally contain sulphuric acids while those with PH
4 generally contain sulphuric acids while those with PH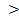 8 contain high percentage of Na+ and thus are alkaline.
8 contain high percentage of Na+ and thus are alkaline.
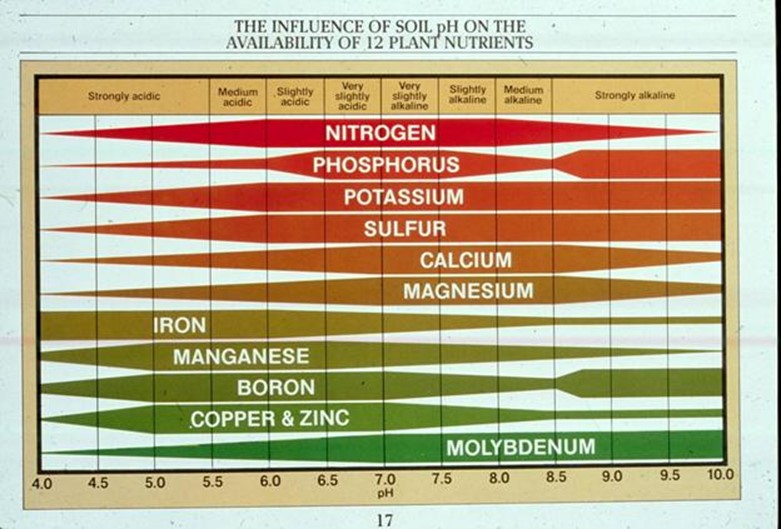
Importance of soil PH
- Most crop plant prefers PH to be between 6 and 7.5 i.e slightly acidic up to slightly alkaline.
- The availability of nutrients to plant depends on soil PH. All plant nutrients are reasonably available between PH 6 and 7.5 which is the optimum PH. The availability of N, P, K, S,Ca, Mg decreases with the increase in soil acidity. Thus acidic soils are often deficient of these nutrients.
edu.uptymez.com
E.g. Nutrient phosphorus is found as phosphate (v) in the soil. At PH  5, the soluble iron, aluminium and manganese react with phosphates (v) to form insoluble complexes and then fix them (makes them unavailable for plants).
5, the soluble iron, aluminium and manganese react with phosphates (v) to form insoluble complexes and then fix them (makes them unavailable for plants).
Fe, Mn and Cu precipitate at high PH. Thus deficiency of these nutrients often limits plants growth in alkaline soils. Manganese and Iron become plant toxic at low PH.
The problem with heavy metals toxic to humans arises near industrial area due to acid rain.
E.g. Ladmium and lead, below PH 4 get dissolved and may enter plant and make crops unfit for human consumption
Conclusion:
Determination of PH of agricultural soil is very important for the selection of suitable crops. Below the PH 4.8 a soil may need liming in order to avoid aluminum toxicity.