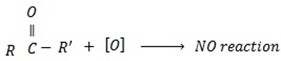NOMENCLATURE OF ALCOHOLS
• Rules of naming alcohols are the same as those alkanes with the following modifications
i. The parent chain must contain hydroxyl group 
ii. In numbering carbons start at the end closer to hydroxyl group.
iii. Position of hydroxyl group should be indicated by using Arabic numerals.
iv. In giving a name of alcohol, the name must end with suffix ol ( after removing e from corresponding name of hydrocarbon)
Example
Give systematic (IUPAC) name of the following organic compounds.

4 – Cyclopropyl- 2 tertbutylpentanol.
OR ( 4- Cyclopropyl – 2- terbuty-pentan-1-ol
Pent – 3 con 1- 01
OR (3 – pentanol).
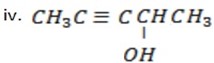
Pent – 3 – yn – 2- ol.
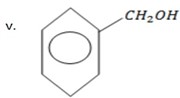
1 – Pheny methanol.
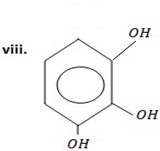
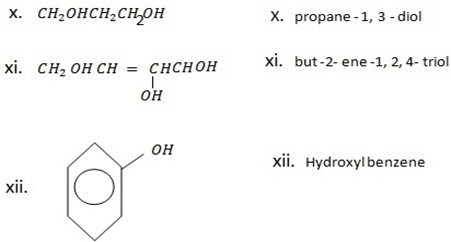
2,3 – dihydroxyl phenol OR (1,2,3,- trihydroxylbenzene)
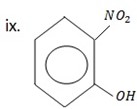
2 – nitrophenol.
PREPARATION OF ALCOHOLS
a) REACTION BETWEEN MOIST SILVER OXIDE AN HALOALKANE.
Generally
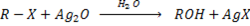
Example
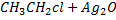
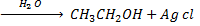
b) REACTION BETWEEN HALOALKANE AND ALKALINE SOLUTION.
• Haloalkane react with alkaline solution like NaOH and KOH yielding alcohol
E.g. In case of NaOH
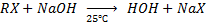
Example
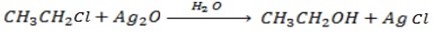
c) ACIDIC HYDROLYSIS OF ALKENE ( REACTION BETWEEN ALKENE AND WATER UNDER PRESENCE OF SULPHURIC ACID).
Generally
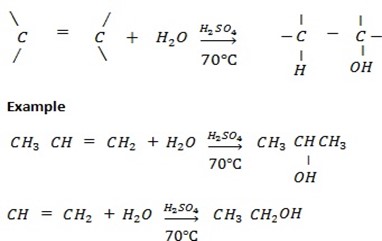
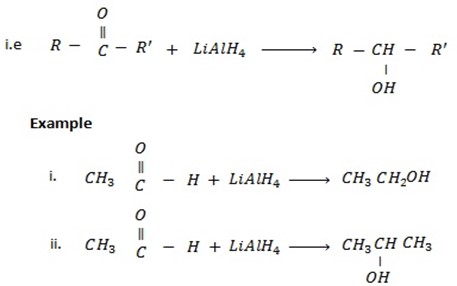
d) REDUCTION OF CARBONYL COMPOUNDS
• Carbonyl compounds react with reducing agent like 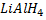 alcohols.
alcohols.
Generally
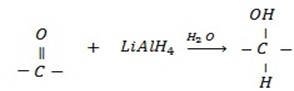
• Aldehydes give primary alcohol.
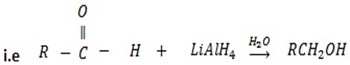
While
• Ketones give secondary alcohol.
e) REDUCTION OF CARBOXYLIC ACID
• Carboxylic acids react with strong reducing agent like 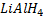 forming primary alcohols.
forming primary alcohols.
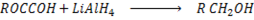
Example
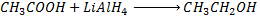
NOTE
In above reaction if 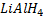 is in limited amount ( or weak reducing agent is used instead of
is in limited amount ( or weak reducing agent is used instead of 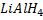 ) aldehyde is formed instead of alcohol.
) aldehyde is formed instead of alcohol.
f) REACTION BETWEEN GRIGINARD REAGENT AND CARBOXYLIC COMPOUNDS ACID FOLLOWED BY ACIDIC HYDROLYSIS
Generally
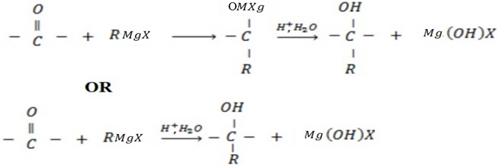
• If the aldehyde used is methanol, primary alcohol is formed.
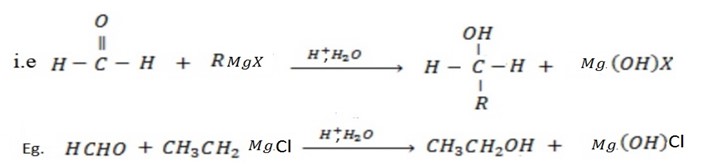
• If higher member of aldehyde is used, secondary alcohol is formed.
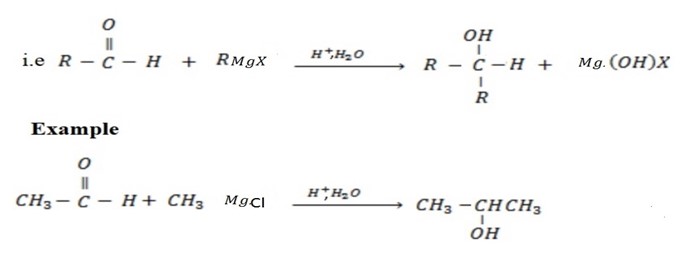
• If the carbonyl compound which is used is Ketone, tertiary alcohol is formed.
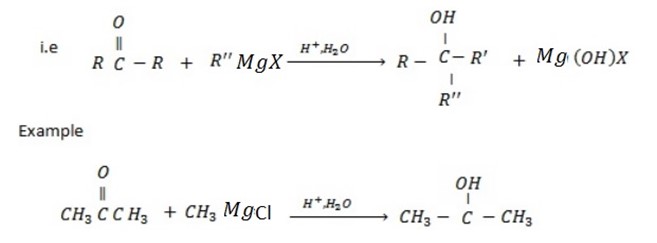
PHYSICAL PROPERTIES OF ALCOHOLS
• Boiling point of alcohol increase with an increase of number of carbon ( As number of carbon increase the molecular weight also increase)
E.g
Boiling point of 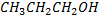 is large than the
is large than the 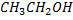
– The less branched alcohol has higher boiling point than that alcohol which is more branched.
Example

– The more branched has lower boiling point due to the following reason,
i. Poor package of carbon atoms
ii. Minimum surface area as it attain more spherical shape hence heating become easier
-In comparison to alcohol and corresponding carbon member of alkene ( with approximately the same molecular weight) alcohol have higher boiling due to presence of hydrogen bond in alcohol.
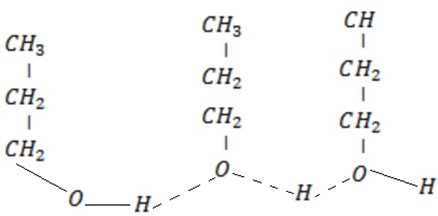

– For polyhydric alcohols. Alcohol with more than one OH– group has the boiling point increase, with number of hydroxyl groups due to increase in position of making hydrogen bond.
Example.
Explain the following the B.P ( boiling point) of ethyl glycol is highest among the compound given below although is little in the molecular weight.
| compound | Formula | Molecular weight | Boiling point in oc |
| Ethylene glycol | 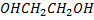 |
62 | 197 |
| Propanol | 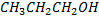 |
60 | 97 |
| Butane | 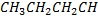 |
58 | -0.5 |
edu.uptymez.com
ANS
Boiling point of alcohol increase with an increase in number of  group.
group.
(for polyhydric alcohol) due to increase in number of position of making hydrogen bonding, ethylene glycol has two  groups so compare to 1- propanol which has only one
groups so compare to 1- propanol which has only one  group. Ethylene glycol, have many position of making hydrogen bonding and hence the boiling will be higher, in case of butane it has group, there is no hydrogen bonding at all that is why has lowest boiling point compound.
group. Ethylene glycol, have many position of making hydrogen bonding and hence the boiling will be higher, in case of butane it has group, there is no hydrogen bonding at all that is why has lowest boiling point compound.
Solubility of alcohol in water decrease with an increase hydrophobic group ( increase number of carbons due to an increase of non polar covalent character).
In increase of polyhydric alcohols solubility increase with an increase in number of  group as a result of increasing number of position of making hydrogen bonding.
group as a result of increasing number of position of making hydrogen bonding.
Example. NECTA 1993 PP1
Explain the solubility of alcohol increase with order
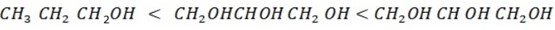
For Polyhydric alcohol solubility increase with an increase in number of position of making hydrogen bonding that is why solubility of given compound increase in that order (i.e the longer the number of group the higher the solubility will be).Alcohol exist as coloured liquid compound because hence easily exposed
EXPLANATION ON COMPARISON OF ACIDIC STRENGTH IN ALCOHOLS AND PHENOLS
Phenol is stronger acid than alcohol due to following reason
1. Easier of releasing hydrogen proton
2. Stability of phenoxide ion formed after releasing hydrogen proton
1. EASIER OF RELEASING HYDROGEN ATOM
Lone pair electrons in oxygen of  groups in phenol are localized they are involved in mesomerism (+M) i.e they are delocalized.
groups in phenol are localized they are involved in mesomerism (+M) i.e they are delocalized.
The delocalization of lone pair electron tends to form +ve charge in oxygen atom of 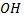 group thus making easier to releasing oxygen proton.
group thus making easier to releasing oxygen proton.
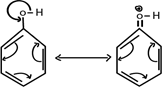
There is no such occasion in alcohol
2. STABILITY OF PHENOXIDE ION FORMED AFTER RELEASING HYDROGEN PROTON
Consider
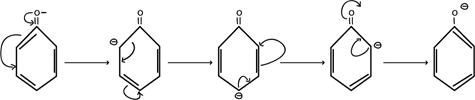
From above mechanism in phenoxide ion, it clearly understood negative charged electrons in oxygen atom of phenoxide is delocalized thus stabilizing the negative charge formed releasing hydrogen proton i.e ( it can exist on its own with combining with hydrogen proton). There is no such occusion alcoxide ion RO– formed in alcohol after releasing hydrogen proton.
ACIDIC THAN ALCOHOL
A. a) REACTION WITH ALKALINE SOLUTION
With alkaline solution like NaOH phenol tend to react with them
Example with 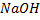 phenol form sodium phenoxide.
phenol form sodium phenoxide.
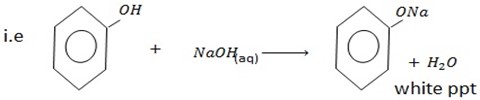
But
There is no such reaction with alcohol indicating that alcohol has basic character and hence phenol is more acidic than alcohol
Alcohol+ alkaline solution 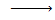 No reaction
No reaction
Note
The reaction is very important in separating phenol from other class of alcohol.
Qn. Without using the distillation explain how would you separate the following pair of organic compounds
i. Phenol and 1- hexanol
ii. Phenol and benzene
B.
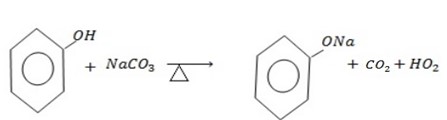
b) REACTION WITH SODIUM CARBONATE
Acids react with carbonate to form carbon dioxide gas phenol like other acids react with sodium carbonate to evolve a gas.
Phenol like other acid react with sodium carbonate evolve a gas which turns lime water milky.
But
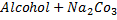
 No reaction
No reaction
c) REACTION WITH CARBOXYLIC ACID
Alcohol react with carboxylic acid in presence of sulphuric to form ester and water.
i.e.
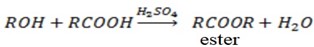
This verify that alcohol has basic character in this case phenol it does not react with carboxylic acid either in presence or in absence of suplhuric acid. This verify that phenol some alcohol character and hence phenol has higher strength than alcohol.

CHEMICAL REACTION OF ALCOHOL
Ø Chemical reaction of alcohol can be classified into the following
a. Reaction which involve replacement of whole OH– group
b. Reactions which involve replacement of H from OH– group
c. Reactions which involve OH– group and β- hydrogen
d. Oxidation
A. REACTION WHICH INVOLVE REPLACEMENT OF HYDROGEN ATOM
Ø Under this heading alcohol react with strong alkaline metals like Li, Na, K to form alkoxide and hydrogen gas is
Ø Generally

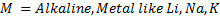
Example

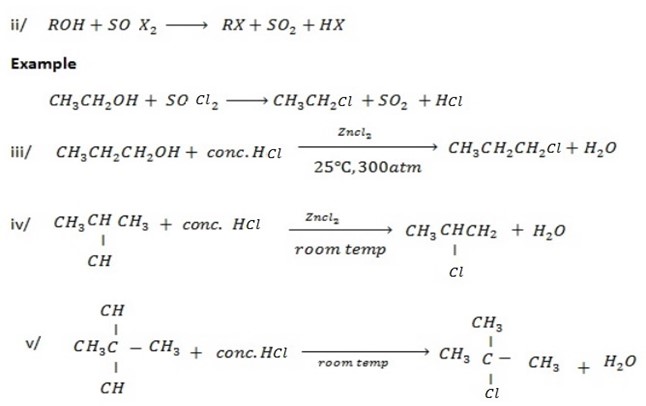
Ø The main reaction under this heading is halogenations of alcohol.
Examples
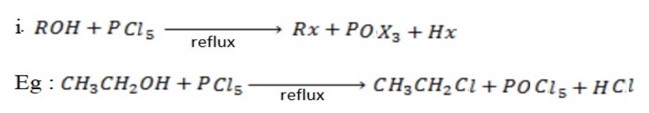
 involved in the above reaction produce denser white fumes with ammonia so that reaction is used as test of presence of
involved in the above reaction produce denser white fumes with ammonia so that reaction is used as test of presence of 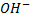 group in the compound.
group in the compound.
B. REACTION WHICH INVOLVE OH– GROUP AND B- HYDROGEN.
Under this heading alcohol undergo elimination reaction to from alkene.
Example
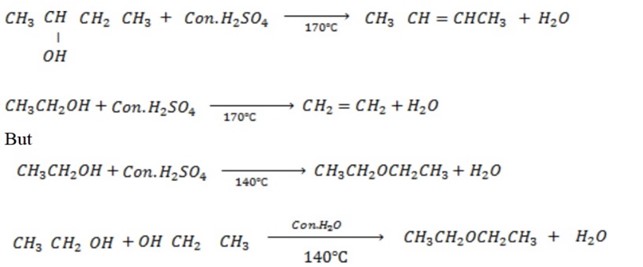
The production of either in above reaction is more favoured when 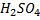 is dilute and cold.
is dilute and cold.
C. OXIDATION
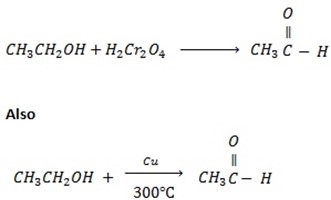
Ø With weak oxidation agent like 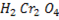 primary alcohol tend to form aldehyde.
primary alcohol tend to form aldehyde.
Example
With strong oxidizing agent like 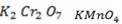 carboxylic acid is formed.
carboxylic acid is formed.
Example
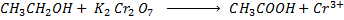
Orange Green

-With either weak oxidizing or strong oxiding agent secondary
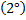 alcohol from Ketone.
alcohol from Ketone.
Generally
NOTE
Tertiary alcohol remit oxidation since there is no hydrogen to be removed.